-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe G.L. Biondi-Zoccai, Marzia Lotrionte, Pierfrancesco Agostoni, Antonio Abbate, Massimiliano Fusaro, Francesco Burzotta, Luca Testa, Imad Sheiban, Giuseppe Sangiorgi, A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50 279 patients at risk for coronary artery disease, European Heart Journal, Volume 27, Issue 22, November 2006, Pages 2667–2674, https://doi.org/10.1093/eurheartj/ehl334
Close - Share Icon Share
Abstract
Aims The role of aspirin in patients with coronary artery disease (CAD) is well established, yet patients happen to discontinue aspirin according to physician’s advice or unsupervised. We thus undertook a systematic review to appraise the hazards inherent to aspirin withdrawal or non-compliance in subjects at risk for or with CAD.
Methods and results Electronic databases were systematically searched (updated January 2006). Study designs, patient characteristics, and outcomes were abstracted. Pooled estimates for odds ratios (OR) were computed according to random-effect methods. From the 612 screened studies, six were selected (50 279 patients). One study (31 750 patients) focused on adherence to aspirin therapy in the secondary prevention of CAD, two studies (2594) on aspirin discontinuation in acute CAD, two studies (13 706) on adherence to aspirin therapy before or shortly after coronary artery bypass grafting, and another (2229) on aspirin discontinuation among patients undergoing drug-eluting stenting. Overall, aspirin non-adherence/withdrawal was associated with three-fold higher risk of major adverse cardiac events (OR=3.14 [1.75–5.61], P=0.0001). This risk was magnified in patients with intracoronary stents, as discontinuation of antiplatelet treatment was associated with an even higher risk of adverse events (OR=89.78 [29.90–269.60]).
Conclusion Non-compliance or withdrawal of aspirin treatment has ominous prognostic implication in subjects with or at moderate-to-high risk for CAD. Aspirin discontinuation in such patients should be advocated only when bleeding risk clearly overwhelms that of atherothrombotic events.
Introduction
Coronary artery disease (CAD) is among the leading causes of morbidity and mortality worldwide, and its impact is likely to increase even further in the next decades.1 Primary, secondary, and tertiary prevention strategies are pivotal to limit the present and future burden of CAD, and in this setting antithrombotic therapy has been established as a mainstay in the management of subjects at risk for CAD, as well as for patients with previous coronary events.2 Aspirin, acetylsalicylic acid, is the most commonly used antiplatelet agent worldwide, given its favourable risk-benefit and cost-benefit profiles.2–4
Although tolerable to most patients, the impact of adverse effects associated with a long-term aspirin regimen is not negligible, especially, given the large number of subjects under aspirin treatment worldwide and the long-term duration of therapy. Although cases of aspirin allergy are known to occur with a low yet predictable rate,5 most adverse effects of aspirin can nonetheless be expected because of its pharmacodynamic properties (inhibition of thromboxane and prostaglandin synthesis), thus translating in increased rates of bleeding (especially from the gastrointestinal tract).6
In light of the potential adverse effects of aspirin, and despite its established beneficial impact, this drug is not uncommonly discontinued by patients, either under physician’s supervision or unsupervised. The latter may be due to prescription errors, lack of compliance, intolerance to adverse effects, or, when occurring under physician guidance, before invasive procedures, or in case of unspecialized care.7 Indeed, spontaneous and unsupervised aspirin discontinuation after 1 year may occur in up to 18% of patients with established CAD, with older age, female gender, lower educational level, and unmarried status as predictors of withdrawal.8
Aspirin withdrawal has been to date incompletely appraised. While potential hazards can be obviously found in interrupting aspirin in subjects who have a clear indication to such therapy, discontinuing this agent might be of value in selected settings if performed for a limited period and under strict supervision. There is, however, a lack of studies explicitly and thoroughly appraising the subject of aspirin withdrawal in patients at risk for or with CAD.
Systematic reviews may provide a thorough and sound appraisal of available evidence, by means of sound search strategies, explicit selection criteria, and formal data abstraction.9,10 Moreover, provided that clinically and statistically homogenous studies are at hand, meta-analytic pooling of individual study findings may be performed, achieving greater statistical power for effect size estimates. Given the uncertainty on the hazards of discontinuing or not adhering to aspirin in patients at risk for or with CAD, we thus undertook a systematic review and meta-analysis of pertinent published studies according to established standards.11
Methods
Search strategy
Electronic database searches were conducted independently by two expert reviewers (G.G.L.B.-Z, M.L.) for pertinent articles published until January 2006 in BioMedCentral (http://www.biomedcentral.com), Google Scholar (http://scholar.google.com), and PubMed (http://www.pubmed.gov). The detailed PubMed search strategy was designed according to established methods (see Appendix).12 Pertinent previous qualitative and systematic reviews were also checked for additional studies.13 Efforts to contact authors were performed to obtain further study details or additional references. Finally, forward and backward snowballing was employed. No language restriction was enforced.
Study selection
Retrieved citations were first screened independently by two reviewers (G.G.L.B.-Z, M.L.) at the title and/or abstract level, with divergences resolved after consensus. If potentially pertinent, they were then appraised as complete reports according to the following explicit selection criteria and with the same appraisal method. Inclusion criteria were: (i) human studies, (ii) reporting on the quantitative appraisal of the cardiovascular risk of aspirin withdrawal or non-compliance, and (iii) in patients at risk for or with established CAD. Exclusion criteria were: (i) non-human setting, (ii) duplicate reporting (in which case the manuscript reporting the largest sample or the longest follow-up was selected), and (iii) inability to compute risk estimates due to case report or series design.
Data abstraction and outcomes
The following data were formally and unblindly abstracted by two independent reviewers on pre-specified forms, with divergences resolved after consensus: authors, journal, years of conduct and publication, study design, sample size, patient characteristics, index diagnosis, prevention stage (primary vs. secondary), coronary lesion characteristics (if available), raw numbers and risks for death, myocardial infarction, stroke, major adverse cardiovascular events (as defined and reported by each investigator), and major bleeding.
Internal validity and quality appraisal
The quality of included studies was appraised according to established methods, by two independent reviewers aware of the study origin and journal, with divergences resolved after consensus.9 Specifically, we separately estimated the risk of selection, performance, detection, and attrition bias (expressed as low risk of bias [A], moderate risk of bias [B], high risk of bias [C], or incomplete reporting leading to inability to ascertain the underlying risk of bias [D]), and abstracted additional design features.9 (Table 1).
Quality appraisal of included studies
| Reference . | Design . | Multicentre . | Selection bias . | Performance bias . | Detection bias . | Attrition bias . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | Prospective cohort | No | A | B | D | A |
| Dacey et al.27 (2000) | Prospective cohort | Yes | A | B | D | A |
| Ferrari et al.30 (2005) | Prospective cohort | No | A | B | B | A |
| Iakovou et al.31 (2005) | Prospective cohort | Yes | A | B | D | A |
| Mangano et al.28 (2002) | Prospective cohort | Yes | A | B | D | A |
| Newby et al.32 (2006) | Prospective cohort | No | A | B | D | B |
| Reference . | Design . | Multicentre . | Selection bias . | Performance bias . | Detection bias . | Attrition bias . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | Prospective cohort | No | A | B | D | A |
| Dacey et al.27 (2000) | Prospective cohort | Yes | A | B | D | A |
| Ferrari et al.30 (2005) | Prospective cohort | No | A | B | B | A |
| Iakovou et al.31 (2005) | Prospective cohort | Yes | A | B | D | A |
| Mangano et al.28 (2002) | Prospective cohort | Yes | A | B | D | A |
| Newby et al.32 (2006) | Prospective cohort | No | A | B | D | B |
The internal validity of included trials was appraised judging the risk for selection, performance, attrition, and adjudication biases, and expressed as low risk of bias (A), moderate risk of bias (B), high risk of bias (C), or incomplete reporting leading to inability to ascertain the underlying risk of bias (D).
Quality appraisal of included studies
| Reference . | Design . | Multicentre . | Selection bias . | Performance bias . | Detection bias . | Attrition bias . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | Prospective cohort | No | A | B | D | A |
| Dacey et al.27 (2000) | Prospective cohort | Yes | A | B | D | A |
| Ferrari et al.30 (2005) | Prospective cohort | No | A | B | B | A |
| Iakovou et al.31 (2005) | Prospective cohort | Yes | A | B | D | A |
| Mangano et al.28 (2002) | Prospective cohort | Yes | A | B | D | A |
| Newby et al.32 (2006) | Prospective cohort | No | A | B | D | B |
| Reference . | Design . | Multicentre . | Selection bias . | Performance bias . | Detection bias . | Attrition bias . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | Prospective cohort | No | A | B | D | A |
| Dacey et al.27 (2000) | Prospective cohort | Yes | A | B | D | A |
| Ferrari et al.30 (2005) | Prospective cohort | No | A | B | B | A |
| Iakovou et al.31 (2005) | Prospective cohort | Yes | A | B | D | A |
| Mangano et al.28 (2002) | Prospective cohort | Yes | A | B | D | A |
| Newby et al.32 (2006) | Prospective cohort | No | A | B | D | B |
The internal validity of included trials was appraised judging the risk for selection, performance, attrition, and adjudication biases, and expressed as low risk of bias (A), moderate risk of bias (B), high risk of bias (C), or incomplete reporting leading to inability to ascertain the underlying risk of bias (D).
Data analysis and synthesis
Continuous variables are reported as mean (standard deviation) or median (range). Categorical variables are expressed as n/N (%). Statistical pooling was performed according to a random-effect model with generic inverse variance weighting, computing risk estimates [expressed according to the individual report as odds ratios (OR), or converted to this as originally reported as relative risks or hazard ratios (HR), with 95% confidence intervals (CI)] by means of the RevMan 4.2 freeware.
Hypothesis testing was set at the two-tailed 0.05 level. A two-tailed 0.10 P-value at χ2 test was considered as cutoff for statistical heterogeneity, whereas I2 values of 25, 50, and 75% were considered to represent, respectively, mild, moderate, and extensive statistical inconsistencies.14 Finally, the small study bias was appraised by graphical inspection of funnel plots and Egger test.
Results
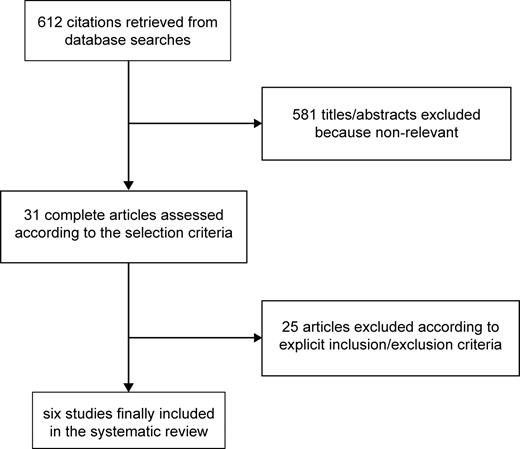
The reviewing process is summarized in Figure 1. Database searches initially retrieved 612 citations, from which 581 hits were excluded at the title or abstract level. After thorough assessment according to the selection criteria, we further excluded 25 citations. Specific reasons for exclusion were: healthy volunteer setting,15 lack of cardiovascular clinical outcomes,7,8,16–19 lack of a control group,20 and inability to provide or lack of risk estimates due to study design.21–25 Another study was excluded because of reporting aspirin substitution with ticlopidine instead of strict aspirin discontinuation.26 We finally included six studies in the analysis27–32(Table 2).
Characteristics of included studies
| Reference . | Years . | Principal investigator . | Location . | Patients . | Focus . | Setting . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | 1999–2002 | G. Montalescot | France | 1 358 | Aspirin discontinuation | Patients admitted for ACS |
| Dacey et al.27 (2000) | 1987–1991 | L. Dacey | USA | 8 641 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Ferrari et al.30 (2005) | 1999–2002 | E. Ferrari | France | 1 236 | Aspirin discontinuation | Patients admitted for ACS |
| Iakovou et al.31 (2005) | 2002–2004 | A. Colombo | Germany, Italy | 2 229 | Aspirin discontinuation | Patients treated with drug-eluting coronary stents |
| Mangano et al.28 (2002) | 1997–2000 | D.T. Mangano | USA | 5 065 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Newby et al.32 (2006) | 1995–2002 | L.K. Newby | USA | 31 750 | Adherence to aspirin treatment | Secondary prevention after diagnosis of CAD |
| Reference . | Years . | Principal investigator . | Location . | Patients . | Focus . | Setting . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | 1999–2002 | G. Montalescot | France | 1 358 | Aspirin discontinuation | Patients admitted for ACS |
| Dacey et al.27 (2000) | 1987–1991 | L. Dacey | USA | 8 641 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Ferrari et al.30 (2005) | 1999–2002 | E. Ferrari | France | 1 236 | Aspirin discontinuation | Patients admitted for ACS |
| Iakovou et al.31 (2005) | 2002–2004 | A. Colombo | Germany, Italy | 2 229 | Aspirin discontinuation | Patients treated with drug-eluting coronary stents |
| Mangano et al.28 (2002) | 1997–2000 | D.T. Mangano | USA | 5 065 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Newby et al.32 (2006) | 1995–2002 | L.K. Newby | USA | 31 750 | Adherence to aspirin treatment | Secondary prevention after diagnosis of CAD |
aOnly combined estimates for subjects continuing aspirin vs. those not on aspirin were provided.
Characteristics of included studies
| Reference . | Years . | Principal investigator . | Location . | Patients . | Focus . | Setting . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | 1999–2002 | G. Montalescot | France | 1 358 | Aspirin discontinuation | Patients admitted for ACS |
| Dacey et al.27 (2000) | 1987–1991 | L. Dacey | USA | 8 641 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Ferrari et al.30 (2005) | 1999–2002 | E. Ferrari | France | 1 236 | Aspirin discontinuation | Patients admitted for ACS |
| Iakovou et al.31 (2005) | 2002–2004 | A. Colombo | Germany, Italy | 2 229 | Aspirin discontinuation | Patients treated with drug-eluting coronary stents |
| Mangano et al.28 (2002) | 1997–2000 | D.T. Mangano | USA | 5 065 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Newby et al.32 (2006) | 1995–2002 | L.K. Newby | USA | 31 750 | Adherence to aspirin treatment | Secondary prevention after diagnosis of CAD |
| Reference . | Years . | Principal investigator . | Location . | Patients . | Focus . | Setting . |
|---|---|---|---|---|---|---|
| Collet et al.21 (2004) | 1999–2002 | G. Montalescot | France | 1 358 | Aspirin discontinuation | Patients admitted for ACS |
| Dacey et al.27 (2000) | 1987–1991 | L. Dacey | USA | 8 641 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Ferrari et al.30 (2005) | 1999–2002 | E. Ferrari | France | 1 236 | Aspirin discontinuation | Patients admitted for ACS |
| Iakovou et al.31 (2005) | 2002–2004 | A. Colombo | Germany, Italy | 2 229 | Aspirin discontinuation | Patients treated with drug-eluting coronary stents |
| Mangano et al.28 (2002) | 1997–2000 | D.T. Mangano | USA | 5 065 | Adherence to aspirin treatment | Patients undergoing CABGa |
| Newby et al.32 (2006) | 1995–2002 | L.K. Newby | USA | 31 750 | Adherence to aspirin treatment | Secondary prevention after diagnosis of CAD |
aOnly combined estimates for subjects continuing aspirin vs. those not on aspirin were provided.
Characteristics of included studies
In a prospective study of 1358 patients admitted for acute myocardial infarction and followed for 1 month, subjects who had recently discontinued aspirin were at significantly higher risk for 1-month death or myocardial infarction, and bleeding, even at multivariable analysis. Infarction occurred 11.9±0.8 days after aspirin withdrawal, and this was in 64% cases primarily a physician’s decision for scheduled surgery.29
Similar data were reported among 1236 patients admitted for acute coronary syndromes (ACS).30 Specifically, aspirin withdrawal was implicated in 4.1% (51 of 1236) of coronary events among the total patients hospitalized for ACS (with 31 non-ST-elevation ACS and 20 ST-elevation ACS), and 13.3% of events among those who relapsed after a previous ACS episode (51 of 383). Moroever, there was a significant association between aspirin discontinuation and presentation with ST-elevation ACS. The delay between aspirin withdrawal and the acute coronary event was 10.0±1.9 days.
Newby et al.32 reported on the long-term outcomes of patients with consistent use of aspirin vs. those without consistent use (thereby including never users, inconsistent users, and those on aspirin but later withdrawing from it) after an established diagnosis of CAD. They found a major protective effect of adherence to aspirin vs. non-adherence on all-cause mortality [HR=0.58 [0.54–0.62)] over a follow-up of 7 years, thus confirming the long-term benefits associated with aspirin use and the risks inherent with aspirin discontinuation or non-compliance.
Iakovou et al.31 showed that, among 2229 patients undergoing percutaneous coronary intervention (PCI) with successful drug-eluting stent implantation, antiplatelet therapy discontinuation was strikingly associated with the risk of death, non-fatal myocardial infarction, or stent thrombosis. Indeed, discontinuation of aspirin and/or thienopyridines (TNP) occurred in 17 patients, leading to a major adverse event in five (29%).
Dacey et al.27 reported on a case–control study performed in 8641 patients undergoing coronary artery bypass grafting (CABG). Aspirin use in the week preceding surgery was associated with a decrease in the risk of in-hospital all-cause death, without any major change in the rate of re-exploration for haemorrhage, amount of chest tube drainage, or blood transfusion.
Mangano et al.28 further confirmed and expanded these findings by showing that aspirin treatment resumed as early as 48 h after CABG led to significant reductions in in-hospital adverse events, including all-cause death, myocardial infarction, stroke, renal failure, mesenteric ischaemia, without any major detrimental effect on wound healing.
Features of the principal excluded studies and reasons for exclusion are presented in Table 3.
Characteristics of main excluded studies
| Reference . | Years . | Principal investigator . | Patients . | Notes and reasons for exclusion . |
|---|---|---|---|---|
| Albaladejo et al.23 (2004) | 1998–2002 | P. Albaladejo | 181 | Retrospective analysis on patients admitted for acute lower limb ischaemia, without any estimate of the risk of aspirin discontinuation |
| Collet et al.21 (2000) | 1992–1996 | J.P. Collet | 475 | Retrospective analysis on patients admitted for acute myocardial infarction, without any estimate of the risk of aspirin discontinuation |
| Kaluza et al.22 (2000) | 1996–1998 | A.E. Raizner | 40 | Case series reporting on adverse clinical events after non-cardiac surgery in patients treated with coronary stents |
| McFadden et al.24 (2004) | 2003–2004 | P.W. Serruys | 4a | Case series reporting on late drug-eluting coronary stent thrombosis |
| Ong et al.25 (2005) | 2002–2003 | P.W. Serruys | 2006a | Cohort study reporting on seven cases of late drug-eluting stent thrombosis (three due to aspirin and TNP withdrawal), without any estimate of the risk of aspirin discontinuation |
| Pascual Figal et al.26 (2000) | 1994–2000 | M. Valdés Chàvarri | 226 | Cohort study reporting on ticlopidine withdrawal in the first 4 weeks after coronary stenting |
| Reference . | Years . | Principal investigator . | Patients . | Notes and reasons for exclusion . |
|---|---|---|---|---|
| Albaladejo et al.23 (2004) | 1998–2002 | P. Albaladejo | 181 | Retrospective analysis on patients admitted for acute lower limb ischaemia, without any estimate of the risk of aspirin discontinuation |
| Collet et al.21 (2000) | 1992–1996 | J.P. Collet | 475 | Retrospective analysis on patients admitted for acute myocardial infarction, without any estimate of the risk of aspirin discontinuation |
| Kaluza et al.22 (2000) | 1996–1998 | A.E. Raizner | 40 | Case series reporting on adverse clinical events after non-cardiac surgery in patients treated with coronary stents |
| McFadden et al.24 (2004) | 2003–2004 | P.W. Serruys | 4a | Case series reporting on late drug-eluting coronary stent thrombosis |
| Ong et al.25 (2005) | 2002–2003 | P.W. Serruys | 2006a | Cohort study reporting on seven cases of late drug-eluting stent thrombosis (three due to aspirin and TNP withdrawal), without any estimate of the risk of aspirin discontinuation |
| Pascual Figal et al.26 (2000) | 1994–2000 | M. Valdés Chàvarri | 226 | Cohort study reporting on ticlopidine withdrawal in the first 4 weeks after coronary stenting |
aFor two cases, duplicate publication is likely.
Characteristics of main excluded studies
| Reference . | Years . | Principal investigator . | Patients . | Notes and reasons for exclusion . |
|---|---|---|---|---|
| Albaladejo et al.23 (2004) | 1998–2002 | P. Albaladejo | 181 | Retrospective analysis on patients admitted for acute lower limb ischaemia, without any estimate of the risk of aspirin discontinuation |
| Collet et al.21 (2000) | 1992–1996 | J.P. Collet | 475 | Retrospective analysis on patients admitted for acute myocardial infarction, without any estimate of the risk of aspirin discontinuation |
| Kaluza et al.22 (2000) | 1996–1998 | A.E. Raizner | 40 | Case series reporting on adverse clinical events after non-cardiac surgery in patients treated with coronary stents |
| McFadden et al.24 (2004) | 2003–2004 | P.W. Serruys | 4a | Case series reporting on late drug-eluting coronary stent thrombosis |
| Ong et al.25 (2005) | 2002–2003 | P.W. Serruys | 2006a | Cohort study reporting on seven cases of late drug-eluting stent thrombosis (three due to aspirin and TNP withdrawal), without any estimate of the risk of aspirin discontinuation |
| Pascual Figal et al.26 (2000) | 1994–2000 | M. Valdés Chàvarri | 226 | Cohort study reporting on ticlopidine withdrawal in the first 4 weeks after coronary stenting |
| Reference . | Years . | Principal investigator . | Patients . | Notes and reasons for exclusion . |
|---|---|---|---|---|
| Albaladejo et al.23 (2004) | 1998–2002 | P. Albaladejo | 181 | Retrospective analysis on patients admitted for acute lower limb ischaemia, without any estimate of the risk of aspirin discontinuation |
| Collet et al.21 (2000) | 1992–1996 | J.P. Collet | 475 | Retrospective analysis on patients admitted for acute myocardial infarction, without any estimate of the risk of aspirin discontinuation |
| Kaluza et al.22 (2000) | 1996–1998 | A.E. Raizner | 40 | Case series reporting on adverse clinical events after non-cardiac surgery in patients treated with coronary stents |
| McFadden et al.24 (2004) | 2003–2004 | P.W. Serruys | 4a | Case series reporting on late drug-eluting coronary stent thrombosis |
| Ong et al.25 (2005) | 2002–2003 | P.W. Serruys | 2006a | Cohort study reporting on seven cases of late drug-eluting stent thrombosis (three due to aspirin and TNP withdrawal), without any estimate of the risk of aspirin discontinuation |
| Pascual Figal et al.26 (2000) | 1994–2000 | M. Valdés Chàvarri | 226 | Cohort study reporting on ticlopidine withdrawal in the first 4 weeks after coronary stenting |
aFor two cases, duplicate publication is likely.
Clinical impact of aspirin withdrawal in included studies
| Reference . | Primary end-point . | Follow-up . | Effect size . | Adjustment . | Other outcomes . |
|---|---|---|---|---|---|
| Collet et al.21 (2004) | All-cause mortality | 1 month | OR=2.05 (1.08–3.89) | Unclear extent, based on univariate analysis | Bleeding |
| Dacey et al.27 (2000) | All-cause mortality | In-hospital | OR=1.82 (1.02–3.23) | Based on univariate analysis and epidemiologic approach | Bleeding |
| Ferrari et al.30 (2005) | ST-segment elevation acute myocardial infarction | NA | Relative risk=2.13 (1.42–3.22) | None performed | NA |
| Iakovou et al.31 (2005) | Sudden cardiac death, post-procedural myocardial infarction not clearly attributable to another coronary lesion, or stent thrombosis | 9 months | OR=89.78 (29.90–269.60)a | Extensive, but with stepwise selection | NA |
| Mangano et al.28 (2002) | All-cause mortality | In-hospital | OR=2.44 (1.61–3.70) | Extensive, but with stepwise selection | Myocardial infarction, stroke, renal failure, wound healing |
| Newby et al.32 (2006) | All-cause mortality | 7 years | HR=1.72 (1.54–2.38)b | Extensive, but with stepwise selection | NA |
| Reference . | Primary end-point . | Follow-up . | Effect size . | Adjustment . | Other outcomes . |
|---|---|---|---|---|---|
| Collet et al.21 (2004) | All-cause mortality | 1 month | OR=2.05 (1.08–3.89) | Unclear extent, based on univariate analysis | Bleeding |
| Dacey et al.27 (2000) | All-cause mortality | In-hospital | OR=1.82 (1.02–3.23) | Based on univariate analysis and epidemiologic approach | Bleeding |
| Ferrari et al.30 (2005) | ST-segment elevation acute myocardial infarction | NA | Relative risk=2.13 (1.42–3.22) | None performed | NA |
| Iakovou et al.31 (2005) | Sudden cardiac death, post-procedural myocardial infarction not clearly attributable to another coronary lesion, or stent thrombosis | 9 months | OR=89.78 (29.90–269.60)a | Extensive, but with stepwise selection | NA |
| Mangano et al.28 (2002) | All-cause mortality | In-hospital | OR=2.44 (1.61–3.70) | Extensive, but with stepwise selection | Myocardial infarction, stroke, renal failure, wound healing |
| Newby et al.32 (2006) | All-cause mortality | 7 years | HR=1.72 (1.54–2.38)b | Extensive, but with stepwise selection | NA |
NA, not applicable or available.
aRisk estimates refer to discontinuation of either aspirin or TNPs, or both.
bOriginally reported for aspirin continuation (HR=0.58 [0.42–0.65]).
Clinical impact of aspirin withdrawal in included studies
| Reference . | Primary end-point . | Follow-up . | Effect size . | Adjustment . | Other outcomes . |
|---|---|---|---|---|---|
| Collet et al.21 (2004) | All-cause mortality | 1 month | OR=2.05 (1.08–3.89) | Unclear extent, based on univariate analysis | Bleeding |
| Dacey et al.27 (2000) | All-cause mortality | In-hospital | OR=1.82 (1.02–3.23) | Based on univariate analysis and epidemiologic approach | Bleeding |
| Ferrari et al.30 (2005) | ST-segment elevation acute myocardial infarction | NA | Relative risk=2.13 (1.42–3.22) | None performed | NA |
| Iakovou et al.31 (2005) | Sudden cardiac death, post-procedural myocardial infarction not clearly attributable to another coronary lesion, or stent thrombosis | 9 months | OR=89.78 (29.90–269.60)a | Extensive, but with stepwise selection | NA |
| Mangano et al.28 (2002) | All-cause mortality | In-hospital | OR=2.44 (1.61–3.70) | Extensive, but with stepwise selection | Myocardial infarction, stroke, renal failure, wound healing |
| Newby et al.32 (2006) | All-cause mortality | 7 years | HR=1.72 (1.54–2.38)b | Extensive, but with stepwise selection | NA |
| Reference . | Primary end-point . | Follow-up . | Effect size . | Adjustment . | Other outcomes . |
|---|---|---|---|---|---|
| Collet et al.21 (2004) | All-cause mortality | 1 month | OR=2.05 (1.08–3.89) | Unclear extent, based on univariate analysis | Bleeding |
| Dacey et al.27 (2000) | All-cause mortality | In-hospital | OR=1.82 (1.02–3.23) | Based on univariate analysis and epidemiologic approach | Bleeding |
| Ferrari et al.30 (2005) | ST-segment elevation acute myocardial infarction | NA | Relative risk=2.13 (1.42–3.22) | None performed | NA |
| Iakovou et al.31 (2005) | Sudden cardiac death, post-procedural myocardial infarction not clearly attributable to another coronary lesion, or stent thrombosis | 9 months | OR=89.78 (29.90–269.60)a | Extensive, but with stepwise selection | NA |
| Mangano et al.28 (2002) | All-cause mortality | In-hospital | OR=2.44 (1.61–3.70) | Extensive, but with stepwise selection | Myocardial infarction, stroke, renal failure, wound healing |
| Newby et al.32 (2006) | All-cause mortality | 7 years | HR=1.72 (1.54–2.38)b | Extensive, but with stepwise selection | NA |
NA, not applicable or available.
aRisk estimates refer to discontinuation of either aspirin or TNPs, or both.
bOriginally reported for aspirin continuation (HR=0.58 [0.42–0.65]).
Meta-analysis
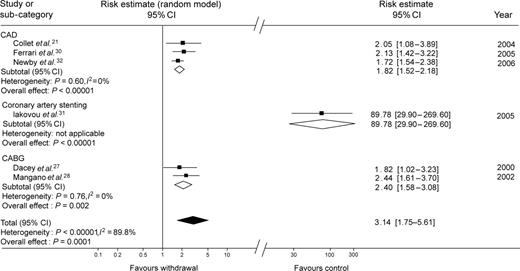
Generic inverse variance weighting enabled statistical pooling according to a random-effect model, providing pooled effect estimates for subgroups and the overall population (Table 4, Figure 2). Specifically, in the subgroup of three studies enrolling patients with ACS or on secondary prevention for CAD, the risk of adverse events for those discontinuing or not adhering to aspirin treatment was increased two-fold (1.82 [1.52–2.18], P for effect <0.00001, P for heterogeneity=0.60, I2=0%). Similarly, combining the two studies focusing on cardiac surgery showed the significant detrimental impact of aspirin discontinuation or non-compliance on the risk of adverse events (2.20 [1.58–3.08], P for effect=0.002, P for heterogeneity=0.76, I2=0%).
Forest plot of the risk of adverse thrombotic events in patients not adhering to or discontinuing aspirin. The analysis is stratified according to the clinical setting and follow-up duration. There is a statistically significant association between aspirin discontinuation and adverse clinical outcomes overall, and in each subgroup. While every subgroup appears clinically and statistically homogeneous, the risk of antiplatelet discontinuation appear far greater after PCI with drug-eluting stent implantation, as reported by Iakovou et al.,31 than in any other study group.
Pooling all these groups yielded a highly significant association between aspirin withdrawal/non-compliance and adverse events (3.14 [1.75–5.61], P for effect=0.0001, P for heterogeneity<0.00001, I2=89.8%). Although such meta-analysis may be considered robust as performed according to a random-effect approach, the presence and extent of statistical heterogeneity limits its conclusiveness and leaves it mainly in the hypothesis-generating realm.
Additional analyses
A major issue is the time between aspirin discontinuation and subsequent adverse events. Whereas this work was not designed to address this topic (already extensively covered by Burger et al.13), pooling available data showed that on an average 10.66 (95% CI 10.25–11.07) days elapsed between drug withdrawal and thrombotic events. These results appear in line with the half-life of platelets, and suggest that in case of mandatory aspirin discontinuation for highly invasive interventions in patients at high risk of bleeding, the drug should be resumed well before that 8–10 days have elapsed.
Testing for publication bias yielded non-significant results (P=0.107), suggesting that among included reports the likelihood of such small study bias was not high. Nonetheless, we should bear in mind the limited number of studies, and the consequent low statistical power for the Egger test.
Sensitivity analyses further confirmed the overall results of our work, as selecting one of the subgroups only, or excluding one study at a time, did not determine major changes in direction or magnitude of statistical findings (all risks >1, P<0.05).
Discussion
Findings of the present systematic review on the hazards of aspirin withdrawal or non-compliance in patients at risk for or with CAD are several-fold: (i) despite a wealth of data on the favourable risk-benefit balance of initiating and maintaining aspirin treatment in these subjects, there are still few observational studies focusing on aspirin discontinuation or non-compliance, and no pertinent controlled randomized trial; (ii) the few available studies addressing the issue of aspirin withdrawal individually clearly demonstrate the relevant hazards inherent in interrupting aspirin treatment in patients in primary or secondary prevention; (iii) meta-analysis combining the available studies further demonstrates the significant increase in the risk of adverse events after an average of 10 days of discontinuing aspirin, across a broad spectrum of patients at risk for or with CAD.
Current clinical and research context
Current data focusing on aspirin discontinuation are still limited and of observational nature. No large randomized trial has been conducted to soundly appraise and define the most appropriate management strategy for patients. Thus, evidence-based recommendations cannot be proposed. Nonetheless, some reports are available suggesting the safety of continuing low-dose aspirin in patients undergoing minor (e.g. oral) surgery.33 French investigators generated guidelines for the peri-operative management of antiplatelet agents,34 reminding that aspirin and non-steroidal anti-inflammatory drugs increase intra- and post-operative bleeding moderately, but do not lead to a significant increase in severe bleeding (e.g. needing transfusion), and that the common practice of withdrawing antiplatelet agents is now challenged because of the increased incidence of thrombotic events in patients in whom treatment was interrupted. They thus recommended that aspirin should not be withdrawn for most vascular procedures and in several additional settings. When a definite increase in intra-operative bleeding is feared, or when surgical haemostasis is difficult, aspirin could be replaced by shorter acting non-steroidal anti-inflammatory drugs, given for a 10-day period and interrupted the day before surgery, and post-operative antiplatelet treatment should be resumed immediately after surgery (first 6 h).
Burger et al.13 have recently reported on a sound and comprehensive systematic review focusing on cardiovascular risks after aspirin peri-operative withdrawal vs. bleeding risks with its continuation. They found that aspirin withdrawal precedes up to 10.2% of acute cardiovascular syndromes, with time intervals between discontinuation and acute cerebral events of 14.3±11.3 days, 8.5±3.6 days for ACS, and 25.8±18.1 days for acute peripheral arterial syndromes. On aspirin-related bleeding risks, 41 studies were retrieved, reporting on 49 590 patients (14 981 on aspirin). Baseline frequency of bleeding complications varied between 0 (skin lesion excision, cataract surgery) and 75% (transrectal prostate biopsy). Whereas aspirin increased the rate of bleeding complications by 1.5, it did not lead to a higher level of the severity of bleeding complications (exception: intracranial surgery, and possibly transurethral prostatectomy). They then concluded that only if low-dose aspirin may cause bleeding risks with increased mortality or sequels comparable with the observed cardiovascular risks after aspirin withdrawal, it should be discontinued prior to an intended operation or procedure.
Pathophysiologically, it is likely that rebound elevations in platelet thromboxane synthesis are among the most important mechanisms underlying the increased thrombotic risk associated with aspirin discontinuation.35 However, experimental in vitro and in vivo studies appraising these issues are still limited, and this field appears as a promising avenue for further research.
Intriguingly, while most commonly used oral antiplatelet agents (i.e. aspirin, ticlopidine, and clopidogrel) may provide similar antithrombotic activities, these agents have important differences in mechanisms of action, safety profile, and cost, which make substitution from one agent to the other not a trivial step in patient management. Indeed, aspirin not only inhibits thromboxane A2 synthesis by irreversibly binding to cyclo-oxygenase, but also promotes other vascular protecting actions, such as the induction of nitric oxide synthesis in neutrophils and endothelial cells.36,37 Moreover, while aspirin ihibits also prostaglandin production in endothelial cells, this action, in contrast to the one on thromboxane production, is short-lived, given the ability of endothelial cells to newly produce proteins and enzymes.38 On the other hand, platelets cannot synthesize proteins, and thus aspirin action on them is irreversible. Thienopyridines have a different mechanism of action (i.e. inhibition of the ADP receptor), and probably much less pleiotropic effects.39 Finally, aspirin, including low-dose aspirin, promotes anti-inflammatory effects and inflammation has been associated with worse outcome in cardiovascular patients.40 Thus, shifting from low-dose aspirin to other antiplatelet agents might not provide the same cardiovascular effects provided by this well-known, affordable, and yet highly effective drug.
Contributions of the present study
This systematic review, while not overcoming the limitations of the individual studies hereby appraised and pooled, further confirms the major detrimental impact of aspirin withdrawal across a large spectrum of subjects at risk for de novo or recurrent cardiovascular events. Indeed, the similar risk faced by patients with acute CAD and those undergoing CABG clearly demonstrates that the beneficial effects of aspirin continuation are not restricted to a specific patient subset. Moreover, the momentous increase in risk faced by those treated with coronary drug-eluting stents can be easily explained by the highly thrombotic milieau generated by PCI with drug-eluting stent implantation, and its consequent dependence on high-intensity antiplatelet regimens for several months.41
Another notable finding of this work is the homogeneity in intervals reported from the several included studies from aspirin discontinuation to thrombotic events. Indeed, the typical average interval of 10 days appears coherent with pathophysiologic data on half platelet population renewal, and has relevant management implications, as in any case of mandatory physician-supervised aspirin withdrawal, the drug should be reinstituted well before these 10 days have elapsed.
Finally, the most relevant result of this scientific endeavour will probably be fulfilled only in the future, if it will be able to guide in the design and conduct of clinically relevant and adequately powered randomized clinical trials capable of establishing the most appropriate treatment approach in patients scheduled for temporary aspirin discontinuation.
Proposal for management of patients on aspirin undergoing invasive procedures
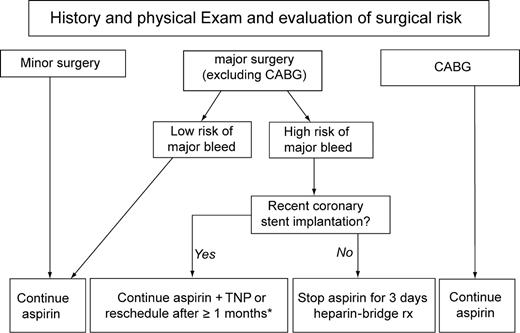
While common clinical sense should guide most decisions concerning the risk-benefit balance of continuing/discontinuing aspirin in patients undergoing invasive procedures, we find that a synthetic flowchart may be potentially useful (Figure 3). In our proposal, aspirin could be continued without major concerns in all patients at low risk of bleeding, as well as those at higher risk of bleeding undergoing minor non-cardiac surgery (e.g. skin surgery) or CABG.
Proposal for management of aspirin treatment in patients at risk for or with CAD undergoing surgical and/or invasive procedures with variable bleeding and thrombotic risks.*The duration of combined antiplatelet therapy with aspirin and TNP, i.e. clopidogrel or ticlopidine, after coronary stent implantation depends on the specific type of stent implanted, and goes from 4 weeks for bare-metal stents to 3 or more months for drug-eluting stents.
For patients scheduled for major cardiac or non-cardiac surgery, we generally advise aspirin discontinuation 3 days before the intervention, with re-institution no later that 3 days afterwards, and using a parenteral anticoagulant drug, such as unfractioned or low-molecular-weight heparin, as bridging antithrombotic treatment.
Such advice for a short-term and bridge use of systemic anticoagulantion in lieu of antiplatelet treatment stems from the established beneficial role of anticoagulant therapy in patients with both acute and chronic coronary syndromes,42,43 and from the shorter half-life of heparins and availability of antagonists for unfractioned heparin (i.e. protamin), in comparison with antiplatelet agents. Even if sound clinical evidence for such an approach is still missing, we find this treatment strategy feasible and potentially effective, while awaiting for prospective randomized trials.
However, patients treated with coronary stents should be managed more carefully, especially if only a few weeks have elapsed since the PCI with stent implantation.22 Thus, we suggest to continue aspirin and TNPs if surgery cannot be postponed until stent endothelialization is completed (≥4 weeks for bare-metal stents, ≥3 months for sirolimus-eluting stents, and ≥6 months for paclitaxel-eluting stents). Afterwards, short-lived aspirin discontinuation with heparin-bridging therapy can be safely envisaged.
Limitations of the present study
Drawbacks of this work are those typical of systematic reviews and meta-analyses of clinical studies,10 but are not limited to them. Pooling of observational studies is considered by several investigators as hypothesis-generating only. In addition, while our findings appear largely homogenous in the specific patient groups identified (Figure 2), overall pooling is fraught by a significant heterogeneity and inconsistency, and should thus be viewed with caution. We should also bear in mind that the included studies largely differed in clinical setting, endpoint definition, and duration of follow-up. Specifically, aspirin withdrawal was the focus of three of the included studies,29–31 whereas ongoing adherence to aspirin treatment was the focus of the other three.27,28,32 Moreover, several reasons may explain aspirin discontinuation (e.g. by doctor’s order, before cardiac or non-cardiac surgical procedures; by doctor’s order, because of side effects with the drug [in which case, generally aspirin is substituted by clopidogrel or ticlopidine]; by the patient, without the doctor’s knowledge). Given the lack of detailed quantitative data in the primary studies, we were unable to analyse the impact of the reasons underlying aspirin interruption on clinical outcomes. Nonetheless given such diversity in the causes of aspirin withdrawal, we might expect varying effects on clinical outcomes in the different clinical settings. Thus, this meta-analysis, while providing intriguing findings, should be considered in light of the other available evidence and placed correctly in the hierarchy of evidence among other observational and non-experimental studies.
Conclusions
This systematic overview suggests that not adhering to or discontinuing aspirin has ominous prognostic implication in subjects with or at moderate-to-high risk for CAD. These findings should be taken into account in the management of patients under such treatment, and aspirin discontinuation or non-compliance should be advocated only when the risk of bleeding or other adverse effects clearly overwhelms that of cardiovascular atherothrombotic events.
Acknowledgements
This study is part of a senior training project of the Center for Overview, Meta-analysis, and Evidence-based medicine Training (COMET), based in Abano Terme, Italy (http://it.geocities.com/comet_milano/Home.htm).
Conflict of interest: None.
Appendix
PubMed was searched according to the strategy modified from Wilczynski and Haynes,12 and incorporating wild cards (identified by *): ((aspirin) AND (therapy OR treatment OR regimen) AND (discontinu* OR interrupt* OR withdraw* OR stop*)) AND (incidence [MeSH:noexp] OR mortality[MeSH Terms] OR follow up studies [MeSH:noexp] OR prognos*[Text Word] OR predict*[Text Word] OR course*[Text Word]).
BioMedCentral and Google Scholar were searched with the following strategy: ((aspirin) AND (therapy OR treatment OR regimen) AND (discontinu* OR interrupt* OR withdraw* OR stop*)) AND (incidence OR mortality OR follow* OR prognos* OR predict* OR course*).