-
PDF
- Split View
-
Views
-
Cite
Cite
Authors/Task Force Members, Adam Torbicki, Arnaud Perrier, Stavros Konstantinides, Giancarlo Agnelli, Nazzareno Galiè, Piotr Pruszczyk, Frank Bengel, Adrian J.B. Brady, Daniel Ferreira, Uwe Janssens, Walter Klepetko, Eckhard Mayer, Martine Remy-Jardin, Jean-Pierre Bassand, Alec Vahanian, John Camm, Raffaele De Caterina, Veronica Dean, Kenneth Dickstein, Gerasimos Filippatos, Christian Funck-Brentano, Irene Hellemans, Steen Dalby Kristensen, Keith McGregor, Udo Sechtem, Sigmund Silber, Michal Tendera, Petr Widimsky, Jose Luis Zamorano, Jose-Luis Zamorano, Felicita Andreotti, Michael Ascherman, George Athanassopoulos, Johan De Sutter, David Fitzmaurice, Tamas Forster, Magda Heras, Guillaume Jondeau, Keld Kjeldsen, Juhani Knuuti, Irene Lang, Mattie Lenzen, Jose Lopez-Sendon, Petros Nihoyannopoulos, Leopoldo Perez Isla, Udo Schwehr, Lucia Torraca, Jean-Luc Vachiery, ESC Committee for Practice Guidelines (CPG), Document Reviewers, Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC), European Heart Journal, Volume 29, Issue 18, September 2008, Pages 2276–2315, https://doi.org/10.1093/eurheartj/ehn310
Close - Share Icon Share
Preamble
Guidelines and Expert Consensus Documents summarize and evaluate all currently available evidence on a particular issue with the aim of assisting physicians in selecting the best management strategies for a typical patient, suffering from a given condition, taking into account the impact on outcome, as well as the risk/benefit ratio of particular diagnostic or therapeutic means. Guidelines are no substitutes for textbooks. The legal implications of medical guidelines have been discussed previously.
A great number of Guidelines and Expert Consensus Documents have been issued in recent years by the European Society of Cardiology (ESC) as well as by other societies and organizations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines and Expert Consensus Documents can be found on the ESC Web Site (http:\\www.escardio.org/guidelines).
In brief, experts in the field are selected and undertake a comprehensive review of the published evidence for management and/or prevention of a given condition. A critical evaluation of diagnostic and therapeutic procedures is performed, including assessment of the risk–benefit ratio. Estimates of expected health outcomes for larger societies are included, where data exist. The level of evidence and the strength of recommendation of particular treatment options are weighed and graded according to predefined scales, as outlined in Tables 1 and 2.
Classes of recommendations
| Class I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective |
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion |
| Class III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful |
| Class I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective |
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion |
| Class III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful |
Classes of recommendations
| Class I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective |
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion |
| Class III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful |
| Class I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective |
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion |
| Class III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful |
Levels of evidence
| Level of evidence A | Data derived from multiple randomized clinical trialsa or meta-analyses |
| Level of evidence B | Data derived from a single randomized clinical triala or large non-randomized studies |
| Level of evidence C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
| Level of evidence A | Data derived from multiple randomized clinical trialsa or meta-analyses |
| Level of evidence B | Data derived from a single randomized clinical triala or large non-randomized studies |
| Level of evidence C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
aOr large accuracy or outcome trial(s) in the case of diagnostic tests or strategies.
Levels of evidence
| Level of evidence A | Data derived from multiple randomized clinical trialsa or meta-analyses |
| Level of evidence B | Data derived from a single randomized clinical triala or large non-randomized studies |
| Level of evidence C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
| Level of evidence A | Data derived from multiple randomized clinical trialsa or meta-analyses |
| Level of evidence B | Data derived from a single randomized clinical triala or large non-randomized studies |
| Level of evidence C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
aOr large accuracy or outcome trial(s) in the case of diagnostic tests or strategies.
The experts of the writing panels have provided disclosure statements of all relationships they may have which might be perceived as real or potential sources of conflicts of interest. These disclosure forms are kept on file at the European Heart House, headquarters of the ESC. Any changes in conflict of interest that arise during the writing period must be notified to the ESC. The Task Force report was entirely supported financially by the European Society of Cardiology and was developed without any involvement of the industry.
The ESC Committee for Practice Guidelines (CPG) supervises and coordinates the preparation of new Guidelines and Expert Consensus Documents produced by Task Forces, expert groups or consensus panels. The Committee is also responsible for the endorsement process of these Guidelines and Expert Consensus Documents or statements. Once the document has been finalized and approved by all the experts involved in the Task Force, it is submitted to outside specialists for review. The document is revised, and finally approved by the CPG and subsequently published.
After publication, dissemination of the message is of paramount importance. Pocket-sized versions and personal digital assistant (PDA)-downloadable versions are useful at the point of care. Some surveys have shown that the intended end-users are sometimes not aware of the existence of guidelines, or simply do not translate them into practice; this is why implementation programmes for new guidelines form an important component of the dissemination of knowledge. Meetings are organized by the ESC and are directed towards its member national societies and key opinion leaders in Europe. Implementation meetings can also be undertaken at national level, once the guidelines have been endorsed by the ESC member societies and translated into the national language. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Thus, the task of writing Guidelines or Expert Consensus Documents covers not only the integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. The loop between clinical research, the writing of guidelines, and implementing them into clinical practice can then only be completed if surveys and registries are performed to verify that real-life daily practice is in keeping with what is recommended in the guidelines. Such surveys and registries also make it possible to evaluate the impact of implementation of the guidelines on patient outcomes. Guidelines and recommendations should help physicians to make decisions in their daily practice; however, the ultimate judgement regarding the care of an individual patient must be made by the physician in charge of that patient's care.
Introduction
Pulmonary embolism (PE) is a relatively common cardiovascular emergency. By occluding the pulmonary arterial bed it may lead to acute life-threatening but potentially reversible right ventricular failure. PE is a difficult diagnosis that may be missed because of non-specific clinical presentation. However, early diagnosis is fundamental, since immediate treatment is highly effective. Depending on the clinical presentation, initial therapy is primarily aimed either at life-saving restoration of flow through occluded pulmonary arteries (PA) or at the prevention of potentially fatal early recurrences. Both initial treatment and the long-term anticoagulation that is required for secondary prevention must be justified in each patient by the results of an appropriately validated diagnostic strategy.1
Epidemiology, predisposing factors, natural history, and the pathophysiology of PE have been described more extensively elsewhere.2–5 This document focuses on currently available and validated methods of diagnosis, prognostic evaluation and therapy of PE. In contrast to previous guidelines, we decided to grade also the level of evidence of diagnostic procedures. The most robust data come from large-scale accuracy or outcome studies. Accuracy studies are designed to establish the characteristics of a diagnostic test (sensitivity and specificity) by comparing test results with a reference diagnostic criterion (the so-called gold standard). Outcome studies evaluate patient outcomes when a given diagnostic test or strategy is used for clinical decision-making. In the field of PE, the outcome measurement is the rate of thromboembolic events [deep vein thrombosis (DVT) or PE] during a 3-month follow-up period in patients left untreated by anticoagulants. The reference for comparison is the rate of DVT or PE in patients left untreated after a negative conventional pulmonary angiogram, which is around 1–2%, with an upper limit of the 95% confidence interval (CI) of 3% during a 3-month follow-up.6 The advantage of outcome studies is that they are easily carried out under normal clinical circumstances and their results are therefore generalizable. However, they do not yield any information on false positives and potential overtreatment. We used the following criteria for grading levels of evidence from diagnostic studies:
Data derived from multiple comparisons or outcome studies or meta-analyses are considered level of evidence A.
Data from a single large comparison or outcome study are considered level of evidence B.
Expert consensus and/or data derived from small comparison or outcome studies are considered level of evidence C.
The first edition of the ESC Clinical Practice Guidelines on PE, published in 2000, was among the documents most often downloaded from the Eur Heart J Web Site.7 We dedicate the current Guidelines to Prof. Henri Denolin, former President of the ESC, Prof. Mireille Brochier, former President of the French Cardiac Society, Prof. Jiri Widimsky, former President of the Czechoslovak Cardiac Society, and Prof. Mario Morpurgo, former Chairman of the ESC Working Group on Pulmonary Circulation, and to other eminent cardiologists who paved the path towards the more effective diagnosis and clinical management of acute pulmonary embolism.
Epidemiology
PE and DVT are two clinical presentations of venous thromboembolism (VTE) and share the same predisposing factors. In most cases PE is a consequence of DVT. Among patients with proximal DVT, about 50% have an associated, usually clinically asymptomatic PE at lung scan.8 In about 70% of patients with PE, DVT can be found in the lower limbs if sensitive diagnostic methods are used.5,9
The epidemiology of VTE has recently been reviewed.4 Although DVT and PE are manifestations of a single disease, namely VTE, PE has features that are distinct from DVT. The risk of death related to the initial acute episode or to recurrent PE is greater in patients who present with PE than in those who present with DVT.10 According to prospective cohort studies, the acute case fatality rate for PE ranges from 7 to 11%.11 Also, recurrent episodes are about three times more likely to be PE after an initial PE than after an initial DVT (about 60% after PE vs. 20% after DVT).11
The prevalence of PE among hospitalized patients in the United States, according to data collected between 1979 and 1999, was 0.4%.12 Though only 40–53 per 100 000 persons were diagnosed with PE per year, the annual incidence in the United States was estimated at 600 000 cases.13 The corresponding figures for Europe are unavailable. Among regional registries, an analysis of 2356 autopsies performed in 1987 on 79% of all deceased inhabitants from the city of Malmo, Sweden, with a population of 230 000, revealed VTE in 595 (25%), while PE was found in 431 (18.3%) of all cases.14 In 308 autopsies (13.1%), PE was considered to be the main cause or a contributory cause of death. The incidence of PE, as diagnosed by lung scintigraphy, within the same period and population was only 48 (2%) cases in the whole Malmo region. From autopsy, phlebography and lung scintigraphy results, the authors estimated the incidence of VTE in the city of Malmo at 42.5/10 000 inhabitants/year. However, recalculation of their data indicates that the incidence of PE was 20.8/10 000 inhabitants/year.14 In a more recent community-based study involving 342 000 inhabitants in Brittany, France, the incidences of VTE and PE were 18.3 and 6.0/10 000/year respectively. However, autopsy data were not available.15 The true incidence of PE is therefore difficult to assess in view of its non-specific clinical presentation.16
Predisposing factors
Although PE can occur in patients without any identifiable predisposing factors, one or more of these factors are usually identified (secondary PE). The proportion of patients with idiopathic or unprovoked PE was about 20% in the International Cooperative Pulmonary Embolism Registry (ICOPER).17
VTE is currently regarded as the result of the interaction between patient-related and setting-related risk factors.18,19 Patient-related predisposing factors are usually permanent, whereas setting-related predisposing factors are more often temporary (Table 3).
Predisposing factors for venous thromboembolism
| Predisposing factor . | Patient-related . | Setting-related . |
|---|---|---|
| Strong predisposing factors (odds ratio >10) | ||
| Fracture (hip or leg) | ✓ | |
| Hip or knee replacement | ✓ | |
| Major general surgery | ✓ | |
| Major trauma | ✓ | |
| Spinal cord injury | ✓ | |
| Moderate predisposing factors (odds ratio 2–9) | ||
| Arthroscopic knee surgery | ✓ | |
| Central venous lines | ✓ | |
| Chemotherapy | ✓ | |
| Chronic heart or respiratory failure | ✓ | |
| Hormone replacement therapy | ✓ | |
| Malignancy | ✓ | |
| Oral contraceptive therapy | ✓ | |
| Paralytic stroke | ✓ | |
| Pregnancy/postpartum | ✓ | |
| Previous VTE | ✓ | |
| Thrombophilia | ✓ | |
| Weak predisposing factors (odds ratio <2) | ||
| Bed rest >3 days | ✓ | |
| Immobility due to sitting (e.g. prolonged car or air travel) | ✓ | |
| Increasing age | ✓ | |
| Laparoscopic surgery (e.g. cholecystectomy) | ✓ | |
| Obesity | ✓ | |
| Pregnancy/antepartum | ✓ | |
| Varicose veins | ✓ | |
| Predisposing factor . | Patient-related . | Setting-related . |
|---|---|---|
| Strong predisposing factors (odds ratio >10) | ||
| Fracture (hip or leg) | ✓ | |
| Hip or knee replacement | ✓ | |
| Major general surgery | ✓ | |
| Major trauma | ✓ | |
| Spinal cord injury | ✓ | |
| Moderate predisposing factors (odds ratio 2–9) | ||
| Arthroscopic knee surgery | ✓ | |
| Central venous lines | ✓ | |
| Chemotherapy | ✓ | |
| Chronic heart or respiratory failure | ✓ | |
| Hormone replacement therapy | ✓ | |
| Malignancy | ✓ | |
| Oral contraceptive therapy | ✓ | |
| Paralytic stroke | ✓ | |
| Pregnancy/postpartum | ✓ | |
| Previous VTE | ✓ | |
| Thrombophilia | ✓ | |
| Weak predisposing factors (odds ratio <2) | ||
| Bed rest >3 days | ✓ | |
| Immobility due to sitting (e.g. prolonged car or air travel) | ✓ | |
| Increasing age | ✓ | |
| Laparoscopic surgery (e.g. cholecystectomy) | ✓ | |
| Obesity | ✓ | |
| Pregnancy/antepartum | ✓ | |
| Varicose veins | ✓ | |
Data are modified from reference 2. This article was published in Circulation, Vol. 107, Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism, I-9–I-16. © (2003) American Heart Association, Inc.
Predisposing factors for venous thromboembolism
| Predisposing factor . | Patient-related . | Setting-related . |
|---|---|---|
| Strong predisposing factors (odds ratio >10) | ||
| Fracture (hip or leg) | ✓ | |
| Hip or knee replacement | ✓ | |
| Major general surgery | ✓ | |
| Major trauma | ✓ | |
| Spinal cord injury | ✓ | |
| Moderate predisposing factors (odds ratio 2–9) | ||
| Arthroscopic knee surgery | ✓ | |
| Central venous lines | ✓ | |
| Chemotherapy | ✓ | |
| Chronic heart or respiratory failure | ✓ | |
| Hormone replacement therapy | ✓ | |
| Malignancy | ✓ | |
| Oral contraceptive therapy | ✓ | |
| Paralytic stroke | ✓ | |
| Pregnancy/postpartum | ✓ | |
| Previous VTE | ✓ | |
| Thrombophilia | ✓ | |
| Weak predisposing factors (odds ratio <2) | ||
| Bed rest >3 days | ✓ | |
| Immobility due to sitting (e.g. prolonged car or air travel) | ✓ | |
| Increasing age | ✓ | |
| Laparoscopic surgery (e.g. cholecystectomy) | ✓ | |
| Obesity | ✓ | |
| Pregnancy/antepartum | ✓ | |
| Varicose veins | ✓ | |
| Predisposing factor . | Patient-related . | Setting-related . |
|---|---|---|
| Strong predisposing factors (odds ratio >10) | ||
| Fracture (hip or leg) | ✓ | |
| Hip or knee replacement | ✓ | |
| Major general surgery | ✓ | |
| Major trauma | ✓ | |
| Spinal cord injury | ✓ | |
| Moderate predisposing factors (odds ratio 2–9) | ||
| Arthroscopic knee surgery | ✓ | |
| Central venous lines | ✓ | |
| Chemotherapy | ✓ | |
| Chronic heart or respiratory failure | ✓ | |
| Hormone replacement therapy | ✓ | |
| Malignancy | ✓ | |
| Oral contraceptive therapy | ✓ | |
| Paralytic stroke | ✓ | |
| Pregnancy/postpartum | ✓ | |
| Previous VTE | ✓ | |
| Thrombophilia | ✓ | |
| Weak predisposing factors (odds ratio <2) | ||
| Bed rest >3 days | ✓ | |
| Immobility due to sitting (e.g. prolonged car or air travel) | ✓ | |
| Increasing age | ✓ | |
| Laparoscopic surgery (e.g. cholecystectomy) | ✓ | |
| Obesity | ✓ | |
| Pregnancy/antepartum | ✓ | |
| Varicose veins | ✓ | |
Data are modified from reference 2. This article was published in Circulation, Vol. 107, Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism, I-9–I-16. © (2003) American Heart Association, Inc.
Patient-related predisposing factors include age, history of previous VTE, active cancer, neurological disease with extremity paresis, medical disorders causing prolonged bed rest, such as heart or acute respiratory failure, and congenital or acquired thrombophilia, hormone replacement therapy and oral contraceptive therapy.
The incidence of VTE increases exponentially with age and this is the case for both idiopathic and secondary PE.14,15 The mean age of patients with acute PE is 62 years; about 65% of patients are aged 60 years or older. Eight-fold higher rates are observed in patients over 80 compared with those younger than 50.20 Identification of the presence and estimation of the relative significance of predisposing factors2 may be helpful both in the assessment of clinical probability for diagnostic purposes and for decisions regarding primary prevention. However, according to a recent survey performed in 358 hospitals across 32 countries, only 58.5 and 39.5% patients at risk of VTE due to medical or surgical causes, respectively, received adequate prophylaxis.21
An association between idiopathic PE and cardiovascular events, including myocardial infarction and stroke, has recently been reported.22,23 Reports of a high risk of PE among obese people, smokers and patients affected by systemic hypertension or metabolic syndrome have renewed interest in the link between arterial thromboembolism and VTE.
Natural history
Since PE in most cases is a consequence of DVT, the natural history of VTE should be considered as a whole instead of looking at DVT and PE separately.
The initial studies on the natural history of VTE were carried out in the setting of orthopaedic surgery during the 1960s.24 A landmark report showed that VTE started during surgery with DVT of the calf in about 30% of patients. DVT resolved spontaneously after a few days in about one-third and did not extend in about 40%, but in 25% it developed into proximal DVT and PE. Since this initial report, knowledge about natural history of VTE has improved.5,20,23,25–31 The evidence suggests that DVT develops less frequently in general than in orthopaedic surgery. The risk of VTE after surgery is highest during the first 2 weeks after surgery but remains elevated for 2–3 months. Antithrombotic prophylaxis significantly reduces the risk of perioperative VTE. The longer the duration of antithrombotic prophylaxis, the lower the incidence of VTE.5,9
Most patients with symptomatic DVT have proximal clots, and in 40–50% of cases this condition is complicated by PE, often without clinical manifestations. Asymptomatic PE is common in the postoperative phase, particularly in patients with asymptomatic DVT who are not given any thromboprophylaxis.5,9
PE occurs 3–7 days after the onset of DVT, and may be fatal within 1 h after the onset of symptoms in 10% of cases, the diagnosis going clinically unrecognized in most fatal cases. PE presents with shock or hypotension in 5–10% of cases, and in up to 50% of cases without shock but with laboratory signs of right ventricular dysfunction (RVD) and/or injury, which indicates a poorer prognosis.32,33 After PE, complete resolution of perfusion defects occurs in about two-thirds of all patients.34 Most deaths (>90%) seem to occur in untreated patients, because of unrecognized PE.35 Fewer than 10% of all deaths were thought to occur in treated patients.5,9,13 Chronic thromboembolic pulmonary hypertension (CTEPH) was found in 0.5–5% of patients with treated PE.5,9,36,37
The frequency of VTE recurrence is identical whatever the initial clinical manifestation of VTE (DVT or PE). It is, however, higher in patients with idiopathic VTE. The risk of fatal PE is higher after a previous episode of isolated DVT, because of the tendency to repeat the initial presentation type in case of subsequent recurrences.10,38 Without anticoagulation about 50% of patients with symptomatic proximal DVT or PE have a recurrence of thrombosis within 3 months.5,9 In patients with previous VTE who had finished their course of at least 3–12 months of anticoagulation treatment, the risk of fatal PE was 0.19–0.49 events per 100 patient-years, depending on the applied diagnostic criteria.38
Pathophysiology
The consequences of acute PE are primarily haemodynamic and become apparent when >30–50% of the pulmonary arterial bed is occluded by thromboemboli.39 The contribution of reflex or humoral pulmonary vasoconstriction, documented in experimental PE, is less important in humans.40–43
Non-thrombotic pulmonary emboli are rare and have different pathophysiological consequences and clinical characteristics (see Non-thrombotic pulmonary embolism).
The key consequences of a pulmonary thromboembolic episode are haemodynamic.32 Large and/or multiple emboli might abruptly increase pulmonary vascular resistance to a level of afterload which cannot be matched by the right ventricle (RV). Sudden death may occur, usually in the form of electromechanical dissociation.44 Alternatively, the patient presents with syncope and/or systemic hypotension, which might progress to shock and death due to acute RV failure. Rightward bulging of the interventricular septum may further compromise systemic cardiac output as a result of diastolic left ventricle (LV) dysfunction.45
In patients surviving the acute embolic episode despite RV failure, systemic sensors activate the sympathetic system. Inotropic and chronotropic stimulation and the Frank–Starling mechanism result in increased pulmonary arterial pressure, which helps to restore resting pulmonary flow, left ventricular filling and output. Together with systemic vasoconstriction, these compensatory mechanisms may stabilize systemic blood pressure.46 This is particularly important because decreased aortic pressure may affect RV coronary perfusion and the function of the RV. However, a non-preconditioned, thin-walled RV is not expected to generate mean pulmonary pressures exceeding 40 mmHg.39
Secondary haemodynamic destabilization may occur, usually within first 24–48 h, as a result of recurrent emboli and/or deterioration of RV function. This may be caused by early recurrences, which are common in undiagnosed or inadequately treated VTE.47 Alternatively, compensatory inotropic and chronotropic stimulation may not suffice to maintain RV function in the long term even in the absence of new embolic episodes. This might be attributable to a potentially detrimental combination of increased RV myocardial oxygen demand and decreased RV coronary perfusion gradient. Both elements contribute to RV ischaemia and dysfunction, and may initiate a vicious circle leading to a fatal outcome.48 Pre-existing cardiovascular disease may influence the efficacy of compensatory mechanisms and consequently affect the prognosis.17
Respiratory insufficiency in PE is predominantly a consequence of haemodynamic disturbances. Several factors may contribute to hypoxia occurring during an episode of PE.49 Low cardiac output results in the desaturation of mixed venous blood entering the pulmonary circulation. Zones of reduced flow and zones of overflow of the capillary bed served by non-obstructed vessels result in ventilation–perfusion mismatch contributing to hypoxaemia. In about one-third of patients, right-to-left shunt through a patent foramen ovale induced by an inverted pressure gradient between the right and left atrium may lead to severe hypoxaemia and an increased risk of paradoxical embolization and stroke.50
Smaller and distal emboli, even though not affecting haemodynamics, may cause areas of alveolar pulmonary haemorrhage, resulting in haemoptysis, pleuritis and usually mild pleural effusion. This clinical presentation is known as ‘pulmonary infarction’. Its effect on gas exchange is usually mild, except in patients with pre-existing cardiorespiratory disease.
Severity of pulmonary embolism
The severity of PE should be understood as an individual estimate of PE-related early mortality risk rather than the anatomical burden and the shape and distribution of intrapulmonary emboli. Therefore, current guidelines suggest replacing potentially misleading terms such as ‘massive’, ‘submassive’ and ‘non-massive’ with the estimated level of the risk of PE-related early death.
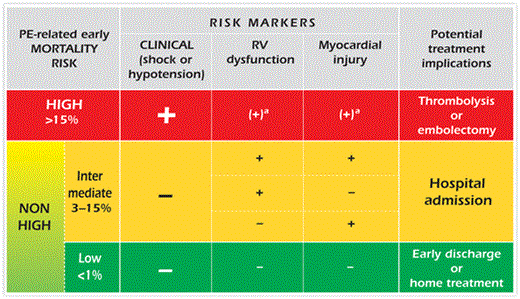
PE can be stratified into several levels of risk of early death (understood as in-hospital or 30-day mortality) based on the presence of risk markers. For practical purposes, risk markers useful for risk stratification in PE can be classified into three groups (Table 4).
Principal markers useful for risk stratification in acute pulmonary embolism
| Clinical markers | Shock |
| Hypotensiona | |
| Markers of RV dysfunction | RV dilatation, hypokinesis or pressure overload on echocardiography |
| RV dilatation on spiral computed tomography | |
| BNP or NT-proBNP elevation | |
| Elevated right heart pressure at RHC | |
| Markers of myocardial injury | Cardiac troponin T or I positiveb |
| Clinical markers | Shock |
| Hypotensiona | |
| Markers of RV dysfunction | RV dilatation, hypokinesis or pressure overload on echocardiography |
| RV dilatation on spiral computed tomography | |
| BNP or NT-proBNP elevation | |
| Elevated right heart pressure at RHC | |
| Markers of myocardial injury | Cardiac troponin T or I positiveb |
BNP = brain natriuretic peptide; NT-proBNP = N-terminal proBNP; RHC = right heart catheterization; RV = right ventricle.
aDefined as a systolic blood pressure <90 mmHg or a pressure drop of ≥40 mmHg for >15 min if not caused by new-onset arrhythmia, hypovolaemia or sepsis.
bHeart-type fatty acid binding protein (H-FABP) is an emerging marker in this category, but still requires confirmation.
Principal markers useful for risk stratification in acute pulmonary embolism
| Clinical markers | Shock |
| Hypotensiona | |
| Markers of RV dysfunction | RV dilatation, hypokinesis or pressure overload on echocardiography |
| RV dilatation on spiral computed tomography | |
| BNP or NT-proBNP elevation | |
| Elevated right heart pressure at RHC | |
| Markers of myocardial injury | Cardiac troponin T or I positiveb |
| Clinical markers | Shock |
| Hypotensiona | |
| Markers of RV dysfunction | RV dilatation, hypokinesis or pressure overload on echocardiography |
| RV dilatation on spiral computed tomography | |
| BNP or NT-proBNP elevation | |
| Elevated right heart pressure at RHC | |
| Markers of myocardial injury | Cardiac troponin T or I positiveb |
BNP = brain natriuretic peptide; NT-proBNP = N-terminal proBNP; RHC = right heart catheterization; RV = right ventricle.
aDefined as a systolic blood pressure <90 mmHg or a pressure drop of ≥40 mmHg for >15 min if not caused by new-onset arrhythmia, hypovolaemia or sepsis.
bHeart-type fatty acid binding protein (H-FABP) is an emerging marker in this category, but still requires confirmation.
Immediate bedside clinical assessment for the presence or absence of clinical markers allows stratification into high-risk and non-high-risk PE (Table 5). This classification should also be applied to patients with suspected PE, as it helps in the choice of the optimal diagnostic strategy and initial management.
Risk stratification according to expected pulmonary embolism-related early mortality rate
 |
 |
aIn the presence of shock or hypotension it is not necessary to confirm RV dysfunction/injury to classify as high risk of PE-related early mortality.
PE = pulmonary embolism; RV = right ventricle.
Risk stratification according to expected pulmonary embolism-related early mortality rate
 |
 |
aIn the presence of shock or hypotension it is not necessary to confirm RV dysfunction/injury to classify as high risk of PE-related early mortality.
PE = pulmonary embolism; RV = right ventricle.
High-risk PE is a life-threatening emergency requiring specific diagnostic and therapeutic strategy (short-term mortality >15%).17,51
Non-high-risk PE can be further stratified according to the presence of markers of RVD and/or myocardial injury into intermediate- and low-risk PE. Intermediate-risk PE is diagnosed if at least one RVD or one myocardial injury marker is positive. Low-risk PE is diagnosed when all checked RVD and myocardial injury markers are found negative (short-term PE-related mortality <1%) [see also Prognostic assessment and Tables A–E in the Supplementary Data. These data show the cutoff values for the key markers of RVD and myocardial injury used in relevant clinical trials which assessed the prognosis of patients with PE].
Diagnosis
Throughout these guidelines and for the purpose of clinical management, ‘confirmed PE’ is understood as a probability of PE high enough to indicate the need for PE-specific treatment and ‘excluded PE’ as a probability of PE low enough to justify withholding specific PE-treatment with an acceptably low risk despite a clinical suspicion of PE. These terms are not meant to indicate absolute certainty regarding the presence or absence of emboli in the pulmonary arterial bed.
Clinical presentation
Evaluating the likelihood of PE in an individual patient according to the clinical presentation is of utmost importance in the interpretation of diagnostic test results and selection of an appropriate diagnostic strategy. In 90% of cases, suspicion of PE is raised by clinical symptoms such as dyspnoea, chest pain and syncope, either singly or in combination. In several series, dyspnoea, tachypnoea, or chest pain were present in more than 90% of patients with PE.52,53 Syncope is a rare but important presentation of PE since it may indicate a severely reduced haemodynamic reserve. In the most severe cases, shock and arterial hypotension may be present. Pleuritic chest pain, whether or not combined with dyspnoea, is one of the most frequent presentations of PE (Table 6). The pain is usually caused by pleural irritation due to distal emboli causing a so-called pulmonary infarction, an alveolar haemorrhage, sometimes accompanied by haemoptysis (54). Isolated dyspnoea of rapid onset is usually due to more central PE causing more prominent haemodynamic consequences than the pulmonary infarction syndrome. It may be associated with retrosternal angina-like chest pain, which may reflect right ventricular ischaemia. Occasionally, the onset of dyspnoea may be very progressive over several weeks, and the diagnosis of PE is evoked by the absence of other classic causes of progressive dyspnoea. In patients with pre-existing heart failure or pulmonary disease, worsening dyspnoea may be the only symptom indicative of PE.
Prevalence of symptoms and signs in patients with suspected PE according to final diagnosis
| . | PE confirmed (n = 219) . | PE excluded (n = 546) . |
|---|---|---|
| Symptoms | ||
| Dyspnoea | 80% | 59% |
| Chest pain (pleuritic) | 52% | 43% |
| Chest pain (substernal) | 12% | 8% |
| Cough | 20% | 25% |
| Haemoptysis | 11% | 7% |
| Syncope | 19% | 11% |
| Signs | ||
| Tachypnoea (≥20/min) | 70% | 68% |
| Tachycardia (>100/min) | 26% | 23% |
| Signs of DVT | 15% | 10% |
| Fever (>38.5°C) | 7% | 17% |
| Cyanosis | 11% | 9% |
| . | PE confirmed (n = 219) . | PE excluded (n = 546) . |
|---|---|---|
| Symptoms | ||
| Dyspnoea | 80% | 59% |
| Chest pain (pleuritic) | 52% | 43% |
| Chest pain (substernal) | 12% | 8% |
| Cough | 20% | 25% |
| Haemoptysis | 11% | 7% |
| Syncope | 19% | 11% |
| Signs | ||
| Tachypnoea (≥20/min) | 70% | 68% |
| Tachycardia (>100/min) | 26% | 23% |
| Signs of DVT | 15% | 10% |
| Fever (>38.5°C) | 7% | 17% |
| Cyanosis | 11% | 9% |
Data are form references 53 and 55.
DVT = deep vein thrombosis.
Prevalence of symptoms and signs in patients with suspected PE according to final diagnosis
| . | PE confirmed (n = 219) . | PE excluded (n = 546) . |
|---|---|---|
| Symptoms | ||
| Dyspnoea | 80% | 59% |
| Chest pain (pleuritic) | 52% | 43% |
| Chest pain (substernal) | 12% | 8% |
| Cough | 20% | 25% |
| Haemoptysis | 11% | 7% |
| Syncope | 19% | 11% |
| Signs | ||
| Tachypnoea (≥20/min) | 70% | 68% |
| Tachycardia (>100/min) | 26% | 23% |
| Signs of DVT | 15% | 10% |
| Fever (>38.5°C) | 7% | 17% |
| Cyanosis | 11% | 9% |
| . | PE confirmed (n = 219) . | PE excluded (n = 546) . |
|---|---|---|
| Symptoms | ||
| Dyspnoea | 80% | 59% |
| Chest pain (pleuritic) | 52% | 43% |
| Chest pain (substernal) | 12% | 8% |
| Cough | 20% | 25% |
| Haemoptysis | 11% | 7% |
| Syncope | 19% | 11% |
| Signs | ||
| Tachypnoea (≥20/min) | 70% | 68% |
| Tachycardia (>100/min) | 26% | 23% |
| Signs of DVT | 15% | 10% |
| Fever (>38.5°C) | 7% | 17% |
| Cyanosis | 11% | 9% |
Data are form references 53 and 55.
DVT = deep vein thrombosis.
Knowledge of which predisposing factors for VTE are present is essential in the evaluation of the likelihood of PE, which increases with the number of predisposing factors present. However, in around 30% of cases PE occurs in the absence of any predisposing factors (unprovoked or idiopathic PE). Individual clinical signs and symptoms are not very helpful, as they are neither sensitive nor specific (Table 6). The chest X-ray is usually abnormal, and the most frequently encountered findings (plate-like atelectasis, pleural effusion or elevation of a hemidiaphragm) are non-specific.56 However, the chest X-ray is very useful in excluding other causes of dyspnoea and chest pain. PE is generally associated with hypoxaemia, but up to 20% of patients with PE have a normal arterial oxygen pressure (PaO2) and a normal alveolar-arterial oxygen gradient [D(A-a)O2].57 Electrocardiographic (ECG) signs of RV strain, such as inversion of T waves in leads V1–V4, a QR pattern in lead V1, the classic S1Q3T3 type and incomplete or complete right bundle-branch block, may be helpful, particularly when of new onset.58,59 Nevertheless, such changes are generally associated with the more severe forms of PE and may be found in right ventricular strain of any cause.
In summary, clinical signs, symptoms and routine laboratory tests do not allow the exclusion or confirmation of acute PE but increase the index of its suspicion.
Assessment of clinical probability
Despite the limited sensitivity and specificity of individual symptoms, signs and common tests, the combination of these variables, either implicitly by the clinician60–63 or by the use of a prediction rule,64–66 makes it possible to discriminate suspected PE patients in categories of clinical or pretest probability corresponding to an increasing prevalence of PE. This has become a key step in all diagnostic algorithms for PE. Indeed, the post-test probability of PE depends not only on the characteristics of the test used but also on pretest probability. Practical implications will be dealt with in further sections.
The value of implicit clinical judgement has been shown in several large series,60–63 one of which was the Prospective Investigation On Pulmonary Embolism Diagnosis (PIOPED).60 There were three main findings of this study: (i) classifying patients into three categories of clinical likelihood of PE is fairly accurate, the prevalence of PE increasing with increasing clinical probability (low, 9%; moderate, 30%; high, 68%); (ii) 90% of patients have a low or moderate (i.e. non-high) clinical probability; and (iii) for an identical result of ventilation–perfusion lung scintigraphy (V/Q scan), the prevalence of PE varies considerably according to the pretest or clinical probability.60
The main limitations of implicit judgement are lack of standardization and the impossibility of teaching it. Therefore, several explicit clinical prediction rules have been developed in the last few years. The most frequently used clinical prediction rule is the Canadian rule, by Wells et al.65 (Table 7). This rule has been validated extensively using both a three-category (low, moderate or high clinical probability) and a two-category scheme (PE likely or unlikely).67–71 It is simple and based on easily collected information. However, the interobserver reproducibility was found to be variable72–74 due to the weight of one subjective item in the rule (alternative diagnosis less likely than PE). The revised Geneva rule is also used in Europe.64 It is simple, based entirely on clinical variables, and standardized. It has also been validated internally and externally,64 although less extensively than the Wells rule. Whichever rule is used, the proportion of patients with PE is around 10% in the low probability category, 30% in the moderate probability category and 65% in the high clinical probability category.
Clinical prediction rules for PE: the Wells score and the revised Geneva score
| Revised Geneva score64 . | Wells score65 . | ||
|---|---|---|---|
| Variable . | Points . | Variable . | Points . |
| Predisposing factors | Predisposing factors | ||
| Age >65 years | +1 | ||
| Previous DVT or PE | +3 | Previous DVT or PE | +1.5 |
| Surgery or fracture within 1 month | +2 | Recent surgery or immobilization | +1.5 |
| Active malignancy | +2 | Cancer | +1 |
| Symptoms | Symptoms | ||
| Unilateral lower limb pain | +3 | ||
| Haemoptysis | +2 | Haemoptysis | +1 |
| Clinical signs | Clinical signs | ||
| Heart rate | Heart rate | ||
| 75–94 beats/min | +3 | >100 beats/min | +1.5 |
| ≥95 beats/min | +5 | ||
| Pain on lower limb deep vein at palpation and unilateral oedema | +4 | Clinical signs of DVT | +3 |
| Clinical judgement | |||
| Alternative diagnosis less likely than PE | +3 | ||
| Clinical probability | Total | Clinical probability (3 levels) | Total |
| Low | 0–3 | Low | 0–1 |
| Intermediate | 4–10 | Intermediate | 2–6 |
| High | ≥11 | High | ≥7 |
| Clinical probability (2 levels) | |||
| PE unlikely | 0–4 | ||
| PE likely | >4 | ||
| Revised Geneva score64 . | Wells score65 . | ||
|---|---|---|---|
| Variable . | Points . | Variable . | Points . |
| Predisposing factors | Predisposing factors | ||
| Age >65 years | +1 | ||
| Previous DVT or PE | +3 | Previous DVT or PE | +1.5 |
| Surgery or fracture within 1 month | +2 | Recent surgery or immobilization | +1.5 |
| Active malignancy | +2 | Cancer | +1 |
| Symptoms | Symptoms | ||
| Unilateral lower limb pain | +3 | ||
| Haemoptysis | +2 | Haemoptysis | +1 |
| Clinical signs | Clinical signs | ||
| Heart rate | Heart rate | ||
| 75–94 beats/min | +3 | >100 beats/min | +1.5 |
| ≥95 beats/min | +5 | ||
| Pain on lower limb deep vein at palpation and unilateral oedema | +4 | Clinical signs of DVT | +3 |
| Clinical judgement | |||
| Alternative diagnosis less likely than PE | +3 | ||
| Clinical probability | Total | Clinical probability (3 levels) | Total |
| Low | 0–3 | Low | 0–1 |
| Intermediate | 4–10 | Intermediate | 2–6 |
| High | ≥11 | High | ≥7 |
| Clinical probability (2 levels) | |||
| PE unlikely | 0–4 | ||
| PE likely | >4 | ||
Clinical prediction rules for PE: the Wells score and the revised Geneva score
| Revised Geneva score64 . | Wells score65 . | ||
|---|---|---|---|
| Variable . | Points . | Variable . | Points . |
| Predisposing factors | Predisposing factors | ||
| Age >65 years | +1 | ||
| Previous DVT or PE | +3 | Previous DVT or PE | +1.5 |
| Surgery or fracture within 1 month | +2 | Recent surgery or immobilization | +1.5 |
| Active malignancy | +2 | Cancer | +1 |
| Symptoms | Symptoms | ||
| Unilateral lower limb pain | +3 | ||
| Haemoptysis | +2 | Haemoptysis | +1 |
| Clinical signs | Clinical signs | ||
| Heart rate | Heart rate | ||
| 75–94 beats/min | +3 | >100 beats/min | +1.5 |
| ≥95 beats/min | +5 | ||
| Pain on lower limb deep vein at palpation and unilateral oedema | +4 | Clinical signs of DVT | +3 |
| Clinical judgement | |||
| Alternative diagnosis less likely than PE | +3 | ||
| Clinical probability | Total | Clinical probability (3 levels) | Total |
| Low | 0–3 | Low | 0–1 |
| Intermediate | 4–10 | Intermediate | 2–6 |
| High | ≥11 | High | ≥7 |
| Clinical probability (2 levels) | |||
| PE unlikely | 0–4 | ||
| PE likely | >4 | ||
| Revised Geneva score64 . | Wells score65 . | ||
|---|---|---|---|
| Variable . | Points . | Variable . | Points . |
| Predisposing factors | Predisposing factors | ||
| Age >65 years | +1 | ||
| Previous DVT or PE | +3 | Previous DVT or PE | +1.5 |
| Surgery or fracture within 1 month | +2 | Recent surgery or immobilization | +1.5 |
| Active malignancy | +2 | Cancer | +1 |
| Symptoms | Symptoms | ||
| Unilateral lower limb pain | +3 | ||
| Haemoptysis | +2 | Haemoptysis | +1 |
| Clinical signs | Clinical signs | ||
| Heart rate | Heart rate | ||
| 75–94 beats/min | +3 | >100 beats/min | +1.5 |
| ≥95 beats/min | +5 | ||
| Pain on lower limb deep vein at palpation and unilateral oedema | +4 | Clinical signs of DVT | +3 |
| Clinical judgement | |||
| Alternative diagnosis less likely than PE | +3 | ||
| Clinical probability | Total | Clinical probability (3 levels) | Total |
| Low | 0–3 | Low | 0–1 |
| Intermediate | 4–10 | Intermediate | 2–6 |
| High | ≥11 | High | ≥7 |
| Clinical probability (2 levels) | |||
| PE unlikely | 0–4 | ||
| PE likely | >4 | ||
In summary, clinical evaluation makes it possible to classify patients into probability categories corresponding to an increasing prevalence of PE, whether assessed by implicit clinical judgement or by a validated prediction rule.
D-dimer
Plasma D-dimer, a degradation product of crosslinked fibrin, has been investigated extensively in recent years.75,76 D-dimer levels are elevated in plasma in the presence of an acute clot because of simultaneous activation of coagulation and fibrinolysis. Hence, a normal D-dimer level renders acute PE or DVT unlikely, i.e. the negative predictive value (NPV) of D-dimer is high. On the other hand, although D-dimer is very specific for fibrin, the specificity of fibrin for VTE is poor because fibrin is produced in a wide variety of conditions, such as cancer, inflammation, infection, necrosis, dissection of the aorta, and the positive predictive value (PPV) of D-dimer is low. Therefore, D-dimer is not useful for confirming PE. There are a number of available assays with different characteristics.75,76 The quantitative enzyme-linked immunoabsorbent assay (ELISA) and ELISA-derived assays have a sensitivity of >95% and a specificity around 40%. They can therefore be used to exclude PE in patients with either a low or a moderate probability of PE. In the emergency department, a negative ELISA D-dimer test can exclude PE without further testing in approximately 30% of patients.63,68,77,78 Outcome studies using the Vidas D-dimer assay showed that the 3-month thromboembolic risk in patients was below 1% in patients left untreated on the basis of a negative test result 63,77–79 (Table 8). Quantitative latex-derived assays and a whole-blood agglutination assay have lower sensitivity, in the range of 85–90%, and are often referred to as moderately sensitive assays.75,76 The most extensively studied to date in outcome studies are the Tinaquant and the SimpliRED assays, which yield a 3-month thromboembolic risk of <1% in patients with a low clinical probability who are left untreated. However, their safety for ruling out PE has not been established in the moderate clinical probability category when using a three-level probability scheme. When using the dichotomous Wells rule, which classifies patients as ‘PE unlikely’ and ‘PE likely’, moderately sensitive assays are safe for the exclusion of PE in patients categorized as PE unlikely, i.e. those with a score of ≤4 points.
Diagnostic yield of various D-dimer assays in excluding acute PE according to outcome studies
| Series . | Clinical probability . | Patients . | D-dimer <500 µg/L . | 3-month thromboembolic risk . |
|---|---|---|---|---|
| . | . | (n) . | [n (%)] . | [% (95% CI)] . |
| Vidas D-dimer63,67,77–79 | Low or moderatea | 3367 | 1184 (33%) | 0.1 (0–0.5) |
| Tinaquant67,80 | Lowa | 2071 | 857 (32%) | 0.6 (0.2–1.4) |
| SimpliRED68 | Low | 930 | 437 (47%) | 0.2 (0–1.3) |
| Series . | Clinical probability . | Patients . | D-dimer <500 µg/L . | 3-month thromboembolic risk . |
|---|---|---|---|---|
| . | . | (n) . | [n (%)] . | [% (95% CI)] . |
| Vidas D-dimer63,67,77–79 | Low or moderatea | 3367 | 1184 (33%) | 0.1 (0–0.5) |
| Tinaquant67,80 | Lowa | 2071 | 857 (32%) | 0.6 (0.2–1.4) |
| SimpliRED68 | Low | 930 | 437 (47%) | 0.2 (0–1.3) |
aPE unlikely in reference 67.
CI = confidence interval.
Diagnostic yield of various D-dimer assays in excluding acute PE according to outcome studies
| Series . | Clinical probability . | Patients . | D-dimer <500 µg/L . | 3-month thromboembolic risk . |
|---|---|---|---|---|
| . | . | (n) . | [n (%)] . | [% (95% CI)] . |
| Vidas D-dimer63,67,77–79 | Low or moderatea | 3367 | 1184 (33%) | 0.1 (0–0.5) |
| Tinaquant67,80 | Lowa | 2071 | 857 (32%) | 0.6 (0.2–1.4) |
| SimpliRED68 | Low | 930 | 437 (47%) | 0.2 (0–1.3) |
| Series . | Clinical probability . | Patients . | D-dimer <500 µg/L . | 3-month thromboembolic risk . |
|---|---|---|---|---|
| . | . | (n) . | [n (%)] . | [% (95% CI)] . |
| Vidas D-dimer63,67,77–79 | Low or moderatea | 3367 | 1184 (33%) | 0.1 (0–0.5) |
| Tinaquant67,80 | Lowa | 2071 | 857 (32%) | 0.6 (0.2–1.4) |
| SimpliRED68 | Low | 930 | 437 (47%) | 0.2 (0–1.3) |
aPE unlikely in reference 67.
CI = confidence interval.
The diagnostic yield of D-dimer relies on its specificity, which varies according to patient characteristics. The specificity of D-dimer in suspected PE decreases steadily with age and may reach ≤10% in patients above 80 years.81 D-dimer is also more frequently elevated in patients with cancer,82,83 in hospitalized patients84 and during pregnancy.85,86 Therefore, the number of patients with suspected PE in whom D-dimer must be measured to exclude one PE (also referred to as the number needed to test) varies between 3 in the emergency department and 10 or above in the specific situations listed above. Deciding whether measuring D-dimer is worthwhile in a given situation remains a matter of clinical judgement.
In summary, a negative D-dimer result in a highly sensitive assay safely excludes PE in patients with a low or moderate clinical probability, while a moderately sensitive assay excludes PE only in patients with a low clinical probability. When using a recently introduced two-level clinical probability assessment scheme, a negative D-dimer result excludes PE safely in PE-unlikely patients either by a highly sensitive or moderately sensitive assay.
Compression ultrasonography and computed tomographic venography
In 90% of patients, PE originates from DVT in a lower limb.87 In a classic study using venography, DVT was found in 70% of patients with proven PE.88 Nowadays, lower limb compression venous ultrasonography (CUS) has largely replaced venography for diagnosing DVT. CUS has a sensitivity over 90% for proximal DVT and a specificity of about 95%.89,90 CUS shows a DVT in 30–50% of patients with PE,89,90 and finding a proximal DVT in patients suspected of PE is sufficient to warrant anticoagulant treatment without further testing.91 In the setting of suspected PE, CUS can be limited to a simple four-point examination (groin and popliteal fossa). The only validated diagnostic criterion for DVT is incomplete compressibility of the vein, which indicates the presence of a clot, whereas flow criteria are unreliable. The diagnostic yield of CUS in suspected PE might be raised by performing complete ultrasonography, including the distal veins. In a recent study, the proportion of patients with PE in whom a DVT could be detected increased from 22% when performing proximal CUS only to 43% using complete CUS, but the specificity decreased accordingly from 96–84%.92 The high specificity of a positive proximal CUS result for PE is confirmed by data from a large prospective outcome study in which 524 patients underwent both multidetector computed tomography (MDCT) and CUS. The sensitivity of CUS for the presence of PE on MSCT was 39% and its specificity was 99%.91 The probability of a positive proximal CUS in suspected PE is higher in patients with leg signs and symptoms than in asymptomatic patients.89,90
More recently, computed tomography (CT) venography has been advocated as a simple way to diagnose DVT in patients with suspected PE as it can be combined with chest CT angiography as a single procedure using only one intravenous injection of contrast dye. In the recent PIOPED II study, combining CT venography with CT angiography increased sensitivity for PE from 83 to 90% and had a similar specificity (around 95%).93,94 However, the corresponding increase in NPV was not clinically significant. Therefore, CT venography increases the overall detection rate only marginally in patients with suspected PE and adds a significant amount of irradiation, which may be a concern, especially in younger women.95
In summary, searching for a proximal DVT in patients with PE by CUS yields a positive result in around 20% of patients. CUS can be used either as a backup procedure to reduce the overall false-negative rate when using single-detector CT (see Diagnostic strategies) or it can be performed to avoid CT when positive in patients with contraindications to contrast dye and/or irradiation. Combining CT venography with CT angiography adds a significant amount of radiation and is not useful when using MDCT.
Ventilation–perfusion scintigraphy
Ventilation–perfusion scintigraphy (V/Q scan) is a robust and well-established diagnostic test for suspected PE. The test has been proved extremely safe to apply and few allergic reactions have been described. The basic principle of the test is based on an intravenous injection of technetium (Tc)-99 m labelled macroaggregated albumin particles, which block a small fraction of pulmonary capillaries and thereby enable scintigraphic assessment of lung perfusion at the tissue level. Where there is occlusion of pulmonary arterial branches, the peripheral capillary bed will not receive particles, rendering the area ‘cold’ on subsequent images. Perfusion scans are combined with ventilation studies, for which multiple tracers, such as xenon (Xe)-133 gas, Tc-99 m labelled aerosols or Tc-99 m-labelled carbon microparticles (Technegas), can be used. The purpose of the additional ventilation scan is to increase specificity by the identification of hypoventilation as a non-embolic cause of hypoperfusion due to reactive vasoconstriction (perfusion–ventilation match). On the contrary, in the case of PE, ventilation is expected to be normal in hypoperfused segments (perfusion–ventilation mismatch).96,97 Traditionally, planar perfusion and ventilation images in at least six projections are acquired. Tc-99 m-labelled ventilation tracers, which (in contrast to the situation in the United States) are approved for clinical use in Europe, are considered preferable to radioactive gases for ventilation imaging because they are deposited in the bronchoalveolar system with little washout, and thus allow the acquisition of multiple projections and more accurate regional matching of perfusion and ventilation.98,99 The radiation exposure from a lung scan with 100 MBq of Tc-99 m macroaggregated albumin particles is 1.1 mSv for an average sized adult according to the International Commission on Radiological Protection (ICRP), and thus significantly lower than that of a spiral CT (2–6 mSv).100 In comparison, a plain chest X-ray delivers a dose of approximately 0.05 mSv.
Lung scan results are frequently classified according to criteria established in the North American PIOPED trial60 into four categories: normal or near-normal, low, intermediate (non-diagnostic) and high probability of PE. The criteria for classification have been a matter of debate and revision.101,102 Nevertheless, the validity of a normal perfusion lung scan has been evaluated in several prospective clinical outcome studies, which observed low event rates,103,104 suggesting that it is a safe practice to withhold anticoagulant therapy in patients with a normal perfusion scan. This has been confirmed recently in a randomized trial comparing the V/Q scan and CT.105 In this large series, 247 patients (35.0%) had normal scan results. Of these, only two patients (0.8%) had proximal DVT on ultrasonography and were treated with anticoagulants. None of the remaining 245 patients had a thromboembolic event during follow-up. Some radiologists accept a single mismatched segmental perfusion defect as indicating a high-probability of PE. Indeed, in a total of 350 patients with at least one segmental perfusion defect and focally normal ventilation, the PPV was 88% (95% CI, 84–91%).60,106–112 This PPV constitutes sufficient proof of the presence of PE to warrant the institution of long-term anticoagulant therapy in most patients. The more stringent PIOPED criteria for a high-probability pattern (two or more mismatched segmental perfusion defects) have a higher PPV for PE and such a result is usually accepted as a confirmation of PE. An analysis from the recent PIOPED II study confirmed the performance of the high-probability V/Q scan for diagnosing PE and of the normal perfusion scan for ruling it out.113 Some centres perform only a perfusion phase and use the chest X-ray as a surrogate for the ventilation study. This is not a preferred strategy when the perfusion scan is not normal, but is acceptable in patients with a normal chest X-ray; any perfusion defect in this situation will be considered a mismatch.114
The high frequency of non-diagnostic intermediate probability scans has been a source of criticism because they indicate the necessity of further diagnostic testing. Multiple strategies to at least partially overcome this problem have been proposed, notably the incorporation of clinical probability,115–117 and data acquisition in tomographic mode.118–120 More recent studies have strongly suggested that data acquisition in tomographic mode as single photon emission computed tomography (SPECT) increases diagnostic accuracy and reduces the frequency of non-diagnostic scans.118–120 SPECT imaging may even allow the use of automated detection algorithms for PE.121
In summary, a normal perfusion scan is very safe for excluding PE. Although less well validated, the combination of a non-diagnostic V/Q scan in a patient with a low clinical probability of PE is an acceptable criterion for excluding PE. A high-probability ventilation–perfusion scan establishes the diagnosis of PE with a high degree of probability, but further tests may be considered in selected patients with a low clinical probability due to the lower PPV of a high-probability V/Q scan result in such patients. In all other combinations of V/Q scan result and clinical probability, further tests should be performed.
Computed tomography
The value of CT angiography for decision-making in suspected PE has changed with recent improvements in the technology available. Two systematic overviews on the performance of single-detector spiral CT in suspected PE reported wide variations regarding both the sensitivity (53–100%) and specificity (73–100%) of CT.122,123 Two large and methodologically robust clinical studies reported a sensitivity around 70% and a specificity of 90% for single-detector CT (SDCT).124,125 The rate of technically inadequate CT angiograms because of motion artefacts or insufficient opacification of the pulmonary vessels was 5–8%. Therefore, a negative SDCT test is not safe for ruling out PE, while the combination of a negative SDCT and the absence of a proximal DVT on lower limb venous ultrasonography in non-high clinical probability patients was associated with a 3-month thromboembolic risk of approximately 1% in two large-scale outcome studies.61,78
Since the introduction of MDCT with high spatial and temporal resolution and quality of arterial opacification, CT angiography has become the method of choice for imaging the pulmonary vasculature for suspected PE in routine clinical practice. It allows adequate visualization of the pulmonary arteries up to at least the segmental level.126–128 Although a sensitivity and specificity for PE above 90% have been reported in an early series,129 the large recent PIOPED II series observed a sensitivity of 83% and a specificity of 96% for MDCT (mainly four-detector).94 Although the choice of the reference diagnostic criteria for PE in the PIOPED II has been criticized, it highlighted the influence of clinical probability on the predictive value of MDCT. In patients with a low or intermediate clinical probability of PE as assessed by the Wells score, a negative CT had a high NPV for PE (96 and 89%, respectively), whereas it was only 60% in those with a high pretest probability. Conversely, the PPV of a positive CT was high (92–96%) in patients with an intermediate or high clinical probability but much lower (58%) in patients with a low pretest likelihood of PE. Therefore, clinicians should be wary in the infrequent situation of discordance between clinical judgement and MDCT result. Four recent studies provide evidence in favour of CT as a stand-alone test to exclude PE. In a prospective management study including 756 consecutive patients referred to the emergency department with a clinical suspicion of PE, all patients with either a high clinical probability or a non-high clinical probability and a positive ELISA D-dimer test underwent both lower limb ultrasonography and MDCT.77 The proportion of patients in whom a proximal DVT was found on ultrasound despite a negative MDCT was only 3/324 (0.9%, 95% CI, 0.3–2.7%).67 In the Christopher Study, all patients classified as PE likely by the dichotomized Wells score and those with a positive D-dimer test underwent a chest MDCT. The 3-month thromboembolic risk in the 1505 patients left untreated because of a negative CT was low (1.1%; 95% CI, 0.6–1.9%).67 Two randomized controlled trials reached similar conclusions. In a Canadian trial comparing V/Q scan and CT (mostly MDCT), only seven of the 531 patients with a negative CT had a DVT and one had a thromboembolic event during follow-up. Hence, the 3-month thromboembolic risk would have been 1.5% (95% CI, 0.8–2.9%) if only CT had been used.105 A European study compared two diagnostic strategies based on D-dimer and MDCT, one with and the other without lower limb CUS.130 In the D-dimer–CT arm, the 3-month thromboembolic risk was 0.3% (95% CI, 0.1–1.2%) among the 627 patients left untreated based on a negative D-dimer or MDCT.
Taken together, these data suggest that a negative MDCT is an adequate criterion for excluding PE in patients with a non-high clinical probability of PE. Whether patients with a negative CT and a high clinical probability should be further investigated by CUS and/or V/Q scintigraphy or pulmonary angiography is controversial. Also, a MDCT showing PE at the segmental or more proximal level is adequate proof of PE in patients with a non-low clinical probability. Since the PPV of MDCT is lower in patients with a low clinical probability of PE (58% in the PIOPED II study),94 further testing should be considered in at least some such patients. As the specificity and PPV of MDCT depend not only on clinical probability but also on the most proximal clot level,94 further testing should be discussed in patients with a low clinical probability and a segmental clot, while treatment could be warranted based on an MDCT showing a thrombus in the lobar or main pulmonary artery.
There has been controversy about the role of CT venography performed in addition to chest CT angiography for diagnosing PE. In the PIOPED II study, the sensitivity of chest CT angiography combined with CT venography was 90% compared with 83% for CT angiography alone.67 However, the absolute gain due to CT venography was modest (detection of 14 additional patients with PE among the 824 patients with a reference diagnosis), reflected by a mere 2% increase in the NPV (97% compared with 95%). CT venography combined with clinical assessment did not yield significantly different predictive values compared with chest CT alone. The lack of clinical usefulness of additional CT venography is compounded by the results of the outcome studies discussed above.67,77 Also, CT venography substantially increases the overall examination radiation, particularly at the pelvic level. Estimates of pelvic radiation vary considerably according to the specific CT venography protocol used. In a study using SDCT, the calculated radiation dose was approximately 2.2 mSv for the chest and 2.5 mSv for the pelvis,131 i.e. twice the radiation dose of a V/Q scan. The gonadal dose for CT venography was two orders of magnitude above that for CT arteriography alone. Interestingly, the analysis of a subgroup of 711 patients from the PIOPED II study who had both venous ultrasonography and CT venography showed a 95.5% concordance between the results of these tests.93 Also, patients with signs or symptoms of DVT were eight times more likely to have DVT and patients with a history of DVT were twice as likely to have positive findings. Therefore, ultrasonography should be used instead of CT venography if indicated (see Diagnostic strategies).
Another controversial area is the clinical significance of isolated subsegmental PE, i.e. the presence of a single subsegmental clot on MDCT, which is found in 1–5% of patients with suspected PE undergoing MDCT.77,132,133 Indeed, the PPV of such a finding is low, and results of outcome studies suggest that such patients left untreated by anticoagulants may have an uneventful course. There may be a role for CUS in this situation in order to ensure that the patient does not have a DVT that would require treatment to assist in decision-making. In a patient without a DVT and with an isolated subsegmental PE, no definitive recommendation can be made because of lack of evidence.
In summary, a SDCT or MDCT showing a thrombus up to the segmental level can be taken as adequate evidence of PE in most instances, whereas the necessity to treat isolated subsegmental thrombi in a patient without a DVT is unclear. In patients with a non-high clinical probability, a negative SDCT must be combined with negative CUS to safely exclude PE, whereas MDCT may be used as a stand-alone test. Whether further testing is mandatory in the rare patients who have a negative MDCT despite a high clinical probability is not settled.
Pulmonary angiography
Pulmonary angiography was refined and was standard practice from the late 1960s onwards.134 The era of digital subtraction angiography has improved image quality. The diagnostic criteria for acute PE in direct angiography were defined almost 40 years ago and consist of direct evidence of a thrombus, either a filling defect or amputation of a pulmonary arterial branch. With direct angiography, thrombi as small as 1 or 2 mm within the subsegmental arteries can be visualized.135 However, there is substantial interobserver variability at the subsegmental level.60 Other indirect signs of PE include the presence of a slow flow of contrast, regional hypoperfusion and delayed or diminished pulmonary venous flow, but these are not validated and hence not diagnostic.
The Miller score in Europe134 and the Walsh score in the United States136 were used to quantify the extent of luminal obstruction. However, with the development and refinement of CT pulmonary angiography, direct pulmonary angiography with contrast injection into the pulmonary arteries is now rarely performed as an isolated diagnostic procedure.
Pulmonary angiography is invasive and not devoid of hazards. The mortality due to pulmonary angiography was 0.2% (95% CI, 0–0.3%) in a pooled analysis of five series with a total of 5696 patients.137 However, the rare deaths attributable to pulmonary angiography occurred in very sick patients with haemodynamic compromise or acute respiratory failure. Although pulmonary angiography has been the gold standard for the diagnosis or exclusion of PE, the technique is now rarely employed because non-invasive CT angiography offers similar or better information. Right ventriculography is difficult to interpret and is now an obsolete technique in the daily practical diagnosis of RVD from acute PE, having been superseded by echocardiography and biomarkers. Moreover, the risk of local bleeding complications is markedly increased if thrombolysis is attempted in patients with PE diagnosed by standard pulmonary angiography.138,139 However, if angiography is done, haemodynamic measurements of pulmonary artery pressure should be recorded.
In summary, pulmonary angiography is a reliable but invasive test and is currently useful when the results of non-invasive imaging are equivocal. Whenever angiography is performed, direct haemodynamic measurements should be performed.
Echocardiography
Right ventricular dilatation is found in at least 25% of patients with PE, and its detection, either by echocardiography or CT, is useful in risk stratification. Echocardiographic criteria used for the diagnosis of PE were different across trials, though usually based on tricuspid insufficiency jet velocity and right ventricular dimensions. Because of the reported sensitivity of around 60–70%, a negative result cannot exclude PE.116,140–145 On the other hand, signs of RV overload or dysfunction may also be due to concomitant cardiac or respiratory disease, in the absence of acute PE.146 Data suggesting that some echocardiographic signs may be more specific are limited.147,148 Three different sets of echocardiographic criteria potentially useful for diagnosing acute PE were compared in a series in which 100 symptomatic patients were enrolled, of whom 62% were referred from the intensive care unit. The criteria which were based either on disturbed RV ejection pattern (the 60–60 sign) or on depressed contractility of the RV free wall compared with its apex (the McConnell sign) seemed to have a higher PPV despite pre-existing cardiorespiratory diseases (Table 9).148 However, concomitant echocardiographic signs of pressure overload are required to prevent the false diagnosis of acute PE in patients with RV free-wall hypo/akinesis due to RV infarction, which may mimic the McConnell sign.149 Tissue Doppler imaging was used to obtain various indices of myocardial performance, which were reported to have a sensitivity of 85–92% and a specificity of 78–92% for PE, but the data are still limited.150
Diagnostic value of three sets of echocardiographic signs suggesting the presence of acute PE in subgroups with and without known previous cardiorespiratory diseases
| . | Patients without known previous cardiorespiratory diseases (n = 46) . | Patients with known previous cardiorespiratory diseases (n = 54) . | ||||
|---|---|---|---|---|---|---|
| . | RV overload criteria . | 60/60 sign . | McConnell sign . | RV overload criteria . | 60/60 sign . | McConnell sign . |
| Specificity (%) | 78 | 100 | 100 | 21 | 89 | 100 |
| Sensitivity (%) | 81 | 25 | 19 | 80 | 26 | 20 |
| PPV (%) | 90 | 100 | 100 | 65 | 82 | 100 |
| NPV (%) | 64 | 37 | 35 | 36 | 40 | 40 |
| . | Patients without known previous cardiorespiratory diseases (n = 46) . | Patients with known previous cardiorespiratory diseases (n = 54) . | ||||
|---|---|---|---|---|---|---|
| . | RV overload criteria . | 60/60 sign . | McConnell sign . | RV overload criteria . | 60/60 sign . | McConnell sign . |
| Specificity (%) | 78 | 100 | 100 | 21 | 89 | 100 |
| Sensitivity (%) | 81 | 25 | 19 | 80 | 26 | 20 |
| PPV (%) | 90 | 100 | 100 | 65 | 82 | 100 |
| NPV (%) | 64 | 37 | 35 | 36 | 40 | 40 |
Data are from reference 148. This article was published in the American Journal of Cardiology, Vol. 90, Kurzyna M, Torbicki A, Pruszczyk P, Burakowska B, Fijalkowska A, Kober J et al., Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism, 507–511. © Elsevier 2002.
RV overload criteria (140): the presence of ≥1 of four signs: (i) right-sided cardiac thrombus; (ii) RV diastolic dimension (parasternal view) >30 mm or a RV/LV ratio >1; (iii) systolic flattening of the interventricular septum; and (iv) acceleration time <90 ms or tricuspid insufficiency pressure gradient >30 mmHg in absence of RV hypertrophy.
The 60/60 sign148 is acceleration time of RV ejection <60 ms in the presence of tricuspid insufficiency pressure gradient ≤ 60 mmHg.
The McConnell sign147 is normokinesia and/or hyperkinesia of the apical segment of the RV free wall despite hypokinesia and/or akinesia of the remaining parts of the RV free wall. Concomitant echocardiographic signs of pressure overload are required to prevent false diagnosis of acute PE in patients with RV free wall hypo/akinesis due to RV infarction.149
PPV = positive predictive value; NPV = negative predictive value.
Diagnostic value of three sets of echocardiographic signs suggesting the presence of acute PE in subgroups with and without known previous cardiorespiratory diseases
| . | Patients without known previous cardiorespiratory diseases (n = 46) . | Patients with known previous cardiorespiratory diseases (n = 54) . | ||||
|---|---|---|---|---|---|---|
| . | RV overload criteria . | 60/60 sign . | McConnell sign . | RV overload criteria . | 60/60 sign . | McConnell sign . |
| Specificity (%) | 78 | 100 | 100 | 21 | 89 | 100 |
| Sensitivity (%) | 81 | 25 | 19 | 80 | 26 | 20 |
| PPV (%) | 90 | 100 | 100 | 65 | 82 | 100 |
| NPV (%) | 64 | 37 | 35 | 36 | 40 | 40 |
| . | Patients without known previous cardiorespiratory diseases (n = 46) . | Patients with known previous cardiorespiratory diseases (n = 54) . | ||||
|---|---|---|---|---|---|---|
| . | RV overload criteria . | 60/60 sign . | McConnell sign . | RV overload criteria . | 60/60 sign . | McConnell sign . |
| Specificity (%) | 78 | 100 | 100 | 21 | 89 | 100 |
| Sensitivity (%) | 81 | 25 | 19 | 80 | 26 | 20 |
| PPV (%) | 90 | 100 | 100 | 65 | 82 | 100 |
| NPV (%) | 64 | 37 | 35 | 36 | 40 | 40 |
Data are from reference 148. This article was published in the American Journal of Cardiology, Vol. 90, Kurzyna M, Torbicki A, Pruszczyk P, Burakowska B, Fijalkowska A, Kober J et al., Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism, 507–511. © Elsevier 2002.
RV overload criteria (140): the presence of ≥1 of four signs: (i) right-sided cardiac thrombus; (ii) RV diastolic dimension (parasternal view) >30 mm or a RV/LV ratio >1; (iii) systolic flattening of the interventricular septum; and (iv) acceleration time <90 ms or tricuspid insufficiency pressure gradient >30 mmHg in absence of RV hypertrophy.
The 60/60 sign148 is acceleration time of RV ejection <60 ms in the presence of tricuspid insufficiency pressure gradient ≤ 60 mmHg.
The McConnell sign147 is normokinesia and/or hyperkinesia of the apical segment of the RV free wall despite hypokinesia and/or akinesia of the remaining parts of the RV free wall. Concomitant echocardiographic signs of pressure overload are required to prevent false diagnosis of acute PE in patients with RV free wall hypo/akinesis due to RV infarction.149
PPV = positive predictive value; NPV = negative predictive value.
Hence, echocardiographic examination is not recommended as an element of elective diagnostic strategy in haemodynamically stable, normotensive patients with suspected PE.116
In patients with suspected high-risk PE presenting with shock or hypotension, the absence of echocardiographic signs of RV overload or dysfunction practically excludes PE as a cause of haemodynamic instability. Furthermore, echocardiography may help in the differential diagnosis of the cause of shock, by detecting cardiac tamponade, acute valvular dysfunction, acute myocardial infarction or hypovolaemia. Conversely, unequivocal signs of RV pressure overload and dysfunction in a haemodynamically compromised patient with suspected PE are highly evocative and may justify aggressive treatment for PE if bedside diagnostic tools must suffice because of the patient's critical condition. In one series, such treatment was introduced in the joint presence of high clinical probability, a shock index ≥1 (defined as heart rate divided by systolic blood pressure) and RVD on echocardiography, and resulted in an acceptable 30-day outcome.151
Concomitant exploration of proximal veins in search of venous clots with compression ultrasound152 and searching for emboli in main pulmonary arteries by transoesophageal echocardiography may be considered in specific clinical situations.153,154 Indeed, because of the high prevalence of bilateral central pulmonary thromboemboli in patients with haemodynamically significant PE, transoesophageal echocardiography may confirm the diagnosis in most cases.155 Also, right heart thrombi, which can be found with transthoracic echocardiography in 4–18% patients with acute PE, justify treatment.156–159
In summary, in a patient with suspected PE who is in a critical condition, bedside echocardiography is particularly helpful in emergency management decisions. In a patient with shock or hypotension, the absence of echocardiographic signs of RV overload or dysfunction practically excludes PE as a cause of haemodynamic compromise. The main role of echocardiography in non-high-risk PE is further prognostic stratification to the intermediate or low-risk category.
Diagnostic strategies
Suspected high-risk and non-high-risk PE are two distinct situations that must be distinguished because the diagnostic strategies differ. Overall, with adequate clinical awareness the prevalence of PE in patients in whom the disease is suspected is low (10–35% in recent large series).67,68,71,77,160 Pulmonary angiography, the definitive standard criterion, is invasive, costly and sometimes difficult to interpret.6,161 Hence, non-invasive diagnostic approaches are warranted, and various combinations of clinical evaluation, plasma D-dimer measurement, lower limb CUS, V/Q lung scintigraphy and, more recently, CT have been evaluated to obviate the requirement for pulmonary angiography. These strategies were applied to patients presenting with suspected PE in the emergency ward,63,68,77,160 during a hospital stay,162 or both.61,67,71 In a recent survey, failure to comply with evidence-based diagnostic strategies when withholding anticoagulation despite the clinical suspicion of PE was related to a significant increase in the number of VTE episodes and in sudden death in the 3 months of follow-up.1 It should be recognized that the approach to suspected PE may legitimately vary according to the local availability of tests in specific clinical settings. The most straightforward diagnostic algorithms for suspected PE are presented in Figures 1 and 2. In contrast, Table 10 provides the information needed to create alternative evidence-based algorithms whenever necessary.
Proposed diagnostic algorithm for patients with suspected high-risk PE, i.e. presenting with shock or hypotension. *CT is considered not immediately available also if the critical condition of a patient allows only bedside diagnostic tests. #Transoesophageal echocardiography may detect thrombi in the pulmonary arteries in a significant proportion of patients with RV overload and PE that is ultimately confirmed by spiral CT; confirmation of DVT with bedside CUS might also help in decision-making.
Proposed diagnostic algorithm for patients with suspected non-high-risk PE (i.e. without shock and hypotension). Two alternative classification schemes may be used to assess clinical probability: a three-level scheme (clinical probability low, intermediate or high) or a two-level scheme (PE unlikely or PE likely). When using a moderately sensitive assay, D-dimer measurement should be restricted to patients with a low clinical probability or a ‘PE unlikely’ classification, while highly sensitive assays may be used in patients with a low or intermediate clinical probability of PE. Plasma D-dimer measurement is of limited use in suspected PE occurring in hospitalized patients. *Anticoagulant treatment for PE. †CT is considered diagnostic of PE if the most proximal thrombus is at least segmental. ‡If single-detector CT is negative, a negative proximal lower limb venous ultrasonography is required in order to safely exclude PE. #If multidetector CT is negative in patients with high clinical probability, further investigation may be considered before withholding PE-specific treatment (see text). PE, pulmonary embolism.
Validated diagnostic criteria for diagnosing PE in patients without shock and hypotension (non-high-risk PE) according to clinical probability
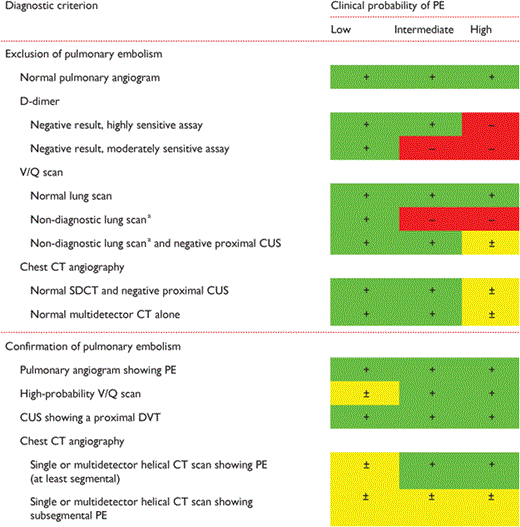 |
 |
Valid criterion (no further testing required), +, green; invalid criterion (further testing necessary), –, red; controversial criterion (further testing to be considered), ±, yellow.
aNon-diagnostic lung scan: low or intermediate probability lung scan according to the PIOPED classification.
CUS = compression venous ultrasonography; DVT = deep venous thrombosis; PE = pulmonary embolism; V/Q scan = ventilation–perfusion scintigraphy.
Validated diagnostic criteria for diagnosing PE in patients without shock and hypotension (non-high-risk PE) according to clinical probability
 |
 |
Valid criterion (no further testing required), +, green; invalid criterion (further testing necessary), –, red; controversial criterion (further testing to be considered), ±, yellow.
aNon-diagnostic lung scan: low or intermediate probability lung scan according to the PIOPED classification.
CUS = compression venous ultrasonography; DVT = deep venous thrombosis; PE = pulmonary embolism; V/Q scan = ventilation–perfusion scintigraphy.
Suspected high-risk pulmonary embolism
Although the greatest body of evidence concerns suspected haemodynamically stable, non-high-risk PE, we have chosen to deal with suspected high-risk PE first because it is an immediately life-threatening situation and patients presenting with shock or hypotension present a distinct clinical problem. The clinical probability is usually high and the differential diagnosis includes cardiogenic shock, acute valvular dysfunction, tamponade and aortic dissection. Hence, the most useful initial test in this situation is echocardiography, which will usually show indirect signs of acute pulmonary hypertension and right ventricular overload if acute PE is the cause of the haemodynamic consequences. Right heart thrombi in transit can be sometimes found on transthoracic echocardiography.156–159 When available, transoesophageal echocardiography may allow direct visualization of a thrombus in the pulmonary artery.153,155,163 However, in a highly unstable patient, or if other tests are not available, the diagnosis of PE may be accepted on the basis of compatible indirect echocardiographic findings alone (Figure 1). If the patient is stabilized by supportive treatment, a definite diagnosis should be sought. Because of the high thrombus load in the pulmonary circulation, CT is usually able to confirm the diagnosis. Conventional pulmonary angiography should be avoided because it carries a risk of mortality in unstable patients161 and increases the risk of bleeding due to thrombolysis.138,139
Suspected non-high-risk pulmonary embolism
Strategy based on computed tomographic angiography
CT angiography has become the main thoracic imaging test for investigating suspected PE.164,165 V/Q scintigraphy remains a validated option but it is less frequently performed because of a high proportion of inconclusive results.60 However, since most patients with suspected PE do not have the disease, CT should not be the first-line test. In patients admitted to the emergency department, plasma D-dimer measurement combined with clinical probability assessment is the logical first step and allows PE to be ruled out in around 30% of patients, with a 3-month thromboembolic risk in patients left untreated below 1% (Table 8).63,67,68,77–80 D-dimer should not be measured in patients with a high clinical probability because of a low NPV in this population.166 It is also less useful in hospitalized patients because the number needed to treat to obtain a clinically relevant negative result is high. In most centres, MDCT is the second-line test in patients with an elevated D-dimer level and the first-line test in patients with a high clinical probability (Figure 2). SDCT or MDCT are considered diagnostic of PE when they show a clot at least at the segmental level of the pulmonary arterial tree. A negative MDCT has been shown to exclude PE safely in several large-scale outcome studies.67,77,167,168 Because of a lower NPV, SDCT must be combined with venous ultrasonography to safely exclude PE.61,78 False-negative results of SDCT61,78 and MDCT94 have been reported in patients with a high clinical probability of PE. However, this situation is infrequent and the 3-month thromboembolic risk is low in such patients.67 Therefore, both the necessity of performing further tests and the nature of these tests in such patients is controversial.
Role of lower limb compression ultrasonography
The role of lower limb CUS is still debated. CUS is mandatory when using SDCT because of its low sensitivity;124,125 indeed, CUS shows a clear DVT in a number of patients with a negative SDCT.61.78 However, most centres are now equipped with MDCT and several large-scale outcome studies have shown that a negative MDCT safely excludes PE, at least in patients with a non-high clinical probability.67,77 Nevertheless, CUS could still be useful when using MDCT. CUS shows a DVT in 30–50% of patients with PE89,90 and finding a proximal DVT in a patients suspected of PE is sufficient to warrant anticoagulant treatment without further testing.91 Hence, performing CUS before CT might be sensible in patients with relative contraindications for CT (renal failure, allergy to contrast dye), so that it can be avoided in patients with a proximal DVT (the specificity for PE of finding a distal DVT is markedly lower).92 CUS might play a role in risk stratification as it has been shown that the presence of a proximal DVT increases the risk of recurrent VTE in patients with PE.169
Role of V/Q scintigraphy
In centres where V/Q scintigraphy is readily available, it remains a valid option for patients with an elevated D-dimer and a contraindication to CT, such as allergy to iodine contrast dye or renal failure. V/Q lung scintigraphy is diagnostic (with either normal or high probability) in approximately 30–50% of emergency ward patients with suspected PE.52,60,62,107 The number of patients with a non-conclusive result may be further reduced by taking clinical probability into account.60 Indeed, patients with a low-probability lung scan and a low clinical probability of PE have a very low prevalence of PE.60,62,116 The NPV of this combination is further reduced by the absence of a DVT on lower limb CUS. In one trial, PE could be excluded by this combination in an additional 24% of patients63 and the 3-month thromboembolic risk of those patients who were left untreated was only 1.7%.62 In an outcome study combining D-dimer, CUS, lung scanning and clinical evaluation, PE could be definitely established or excluded in 89% of the study patients.63 In a recent randomized trial comparing two diagnostic strategies, 99% of patients could be safely managed without pulmonary angiography or CT by a combination of V/Q scan, clinical probability and CUS (initial CUS in all patients and repeat CUS at 1 week in selected patients).105 Only 6 of 611 patients (1.0%, 95% CI, 0.5–2.1%) in whom PE was excluded developed VTE during follow-up. The yield of repeat CUS was very low (one DVT out of 78 examinations).105
| Recommendations: diagnosis . | Classa . | Levelb . |
|---|---|---|
| Suspected high-risk PE | ||
| • In high-risk PE, as indicated by the presence of shock or hypotension, emergency CT or bedside echocardiography (depending on availability and clinical circumstances) is recommended for diagnostic purposes | I | C |
| Suspected non-high-risk PE | ||
| • In non-high-risk PE, basing the diagnostic strategy on clinical probability assessed either implicitly or using a validated prediction rule is recommended | I | A |
| • Plasma D-dimer measurement is recommended in emergency department patients to reduce the need for unnecessary imaging and irradiation, preferably using a highly sensitive assay | I | A |
| • Lower limb CUS in search of DVT may be considered in selected patients with suspected PE to obviate the need for further imaging tests if the result is positive | IIb | B |
| • Systematic use of echocardiography for diagnosis in haemodynamically stable, normotensive patients is not recommended | III | C |
| • Pulmonary angiography should be considered when there is discrepancy between clinical evaluation and results of non-invasive imaging tests | IIa | C |
| • The use of validated criteria for diagnosing PE is recommended. Validated criteria according to clinical probability of PE (low, intermediate or high) are detailed below (see also Table 10) | I | B |
| Suspected non-high-risk PE | ||
| Low clinical probability | ||
| • Normal D-dimer level using either a highly or moderately sensitive assay excludes PE | I | A |
| • Normal perfusion lung scintigraphy excludes PE | I | A |
| • Non-diagnostic (low or intermediate probability) V/Q scan may exclude PE | IIa | B |
| particularly when combined with negative proximal CUS | I | A |
| • Negative MDCT safely excludes PE | I | A |
| • Negative SDCT only excludes PE when combined with negative proximal CUS | I | A |
| • High-probability V/Q scan may confirm PE but … | IIa | B |
| further testing may be considered in selected patients to confirm PE | IIb | B |
| • CUS showing a proximal DVT confirms PE | I | B |
| • If CUS shows only a distal DVT, further testing should be considered to confirm PE | IIa | B |
| • SDCT or MDCT showing a segmental or more proximal thrombus confirms PE | I | A |
| • Further testing should be considered to confirm PE if SDCT or MDCT shows only subsegmental clots | IIa | B |
| Suspected non-high-risk PE | ||
| Intermediate clinical probability | ||
| • Normal D-dimer level using a highly sensitive assay excludes PE | I | A |
| • Further testing should be considered if D-dimer level is normal when using a less sensitive assay | IIa | B |
| • Normal perfusion lung scintigraphy excludes PE | I | A |
| • In case of a non-diagnostic V/Q scan, further testing is recommended to exclude or confirm PE | I | B |
| • Negative MDCT excludes PE | I | A |
| • Negative SDCT only excludes PE when combined with negative proximal CUS | I | A |
| • High-probability ventilation–perfusion lung scintigraphy confirms PE | I | A |
| • CUS showing a proximal DVT confirms PE | I | B |
| • If CUS shows only a distal DVT, further testing should be considered | IIa | B |
| • SDCT or MDCT showing a segmental or more proximal thrombus confirms PE | I | A |
| • Further testing may be considered in case of subsegmental clots to confirm PE | IIb | B |
| Suspected non-high-risk PE | ||
| High clinical probability | ||
| • D-dimer measurement is not recommended in high clinical probability patients as a normal result does not safely exclude PE even when using a highly sensitive assay | III | C |
| • In patients with a negative CT, further tests should be considered in selected patients to exclude PE | IIa | B |
| • High-probability ventilation–perfusion lung scintigraphy confirms PE | I | A |
| • CUS showing a proximal DVT confirms PE | I | B |
| • If CUS shows only a distal DVT, further testing should be considered | IIb | B |
| • SDCT or MDCT showing a segmental or more proximal thrombus confirms PE | I | A |
| • Further testing may be considered where there are subsegmental clots, to confirm PE | IIb | B |
| Recommendations: diagnosis . | Classa . | Levelb . |
|---|---|---|
| Suspected high-risk PE | ||
| • In high-risk PE, as indicated by the presence of shock or hypotension, emergency CT or bedside echocardiography (depending on availability and clinical circumstances) is recommended for diagnostic purposes | I | C |
| Suspected non-high-risk PE | ||
| • In non-high-risk PE, basing the diagnostic strategy on clinical probability assessed either implicitly or using a validated prediction rule is recommended | I | A |
| • Plasma D-dimer measurement is recommended in emergency department patients to reduce the need for unnecessary imaging and irradiation, preferably using a highly sensitive assay | I | A |
| • Lower limb CUS in search of DVT may be considered in selected patients with suspected PE to obviate the need for further imaging tests if the result is positive | IIb | B |
| • Systematic use of echocardiography for diagnosis in haemodynamically stable, normotensive patients is not recommended | III | C |
| • Pulmonary angiography should be considered when there is discrepancy between clinical evaluation and results of non-invasive imaging tests | IIa | C |
| • The use of validated criteria for diagnosing PE is recommended. Validated criteria according to clinical probability of PE (low, intermediate or high) are detailed below (see also Table 10) | I | B |
| Suspected non-high-risk PE | ||
| Low clinical probability | ||
| • Normal D-dimer level using either a highly or moderately sensitive assay excludes PE | I | A |
| • Normal perfusion lung scintigraphy excludes PE | I | A |
| • Non-diagnostic (low or intermediate probability) V/Q scan may exclude PE | IIa | B |
| particularly when combined with negative proximal CUS | I | A |
| • Negative MDCT safely excludes PE | I | A |
| • Negative SDCT only excludes PE when combined with negative proximal CUS | I | A |
| • High-probability V/Q scan may confirm PE but … | IIa | B |
| further testing may be considered in selected patients to confirm PE | IIb | B |
| • CUS showing a proximal DVT confirms PE | I | B |
| • If CUS shows only a distal DVT, further testing should be considered to confirm PE | IIa | B |
| • SDCT or MDCT showing a segmental or more proximal thrombus confirms PE | I | A |
| • Further testing should be considered to confirm PE if SDCT or MDCT shows only subsegmental clots | IIa | B |
| Suspected non-high-risk PE | ||
| Intermediate clinical probability | ||
| • Normal D-dimer level using a highly sensitive assay excludes PE | I | A |
| • Further testing should be considered if D-dimer level is normal when using a less sensitive assay | IIa | B |
| • Normal perfusion lung scintigraphy excludes PE | I | A |
| • In case of a non-diagnostic V/Q scan, further testing is recommended to exclude or confirm PE | I | B |
| • Negative MDCT excludes PE | I | A |
| • Negative SDCT only excludes PE when combined with negative proximal CUS | I | A |
| • High-probability ventilation–perfusion lung scintigraphy confirms PE | I | A |
| • CUS showing a proximal DVT confirms PE | I | B |
| • If CUS shows only a distal DVT, further testing should be considered | IIa | B |
| • SDCT or MDCT showing a segmental or more proximal thrombus confirms PE | I | A |
| • Further testing may be considered in case of subsegmental clots to confirm PE | IIb | B |
| Suspected non-high-risk PE | ||
| High clinical probability | ||
| • D-dimer measurement is not recommended in high clinical probability patients as a normal result does not safely exclude PE even when using a highly sensitive assay | III | C |
| • In patients with a negative CT, further tests should be considered in selected patients to exclude PE | IIa | B |
| • High-probability ventilation–perfusion lung scintigraphy confirms PE | I | A |
| • CUS showing a proximal DVT confirms PE | I | B |
| • If CUS shows only a distal DVT, further testing should be considered | IIb | B |
| • SDCT or MDCT showing a segmental or more proximal thrombus confirms PE | I | A |
| • Further testing may be considered where there are subsegmental clots, to confirm PE | IIb | B |
aClass of recommendation.
bLevel of evidence.
CUS = compression venous ultrasonography.
Role of echocardiography
Echocardiography does not play a major part in detecting suspected non-high-risk PE. Indeed, it has a limited sensitivity (around 60–70%)116,143–145 and a negative echocardiogram does not allow the exclusion of PE. Its specificity is around 90% and an echocardiogram showing signs of right ventricular dysfunction in a patient with a moderate or high clinical probability of PE would theoretically yield a post-test probability of PE high enough to consider the diagnosis confirmed.116,143–145 However, most clinicians would probably require more direct evidence of a clot, either in the lower limbs or in the pulmonary arteries, to confirm the diagnosis before deciding on several months of anticoagulant treatment. Therefore, the main role for echocardiography in non-high-risk PE is prognostic stratification to the intermediate or low risk category.
Areas of uncertainty
Despite considerable progress in PE diagnosis, several areas of uncertainty persist. The diagnostic value and clinical significance of a single subsegmental defect on MDCT are still debated.170 Therefore, deciding between further investigations, treatment or abstention should be made on an individual basis. Likewise, although false-negative MDCT examinations are reported in patients with a high clinical probability,94 it is unclear whether they should be submitted to further tests. In particular, pulmonary angiography is no longer unanimously considered as the gold standard for PE. The role and cost-effectiveness of CUS in suspected PE should be further clarified.
Prognostic assessment
Clinical assessment of haemodynamic status
Hypotension and shock
The existing evidence regarding the prognostic significance of shock and hypotension in acute PE has been reviewed recently.33 It is mostly derived from observational studies such as the ICOPER and Management and Prognosis in Pulmonary Embolism Trial (MAPPET) registry.17,51 In a post hoc analysis of ICOPER data, the 90-day all-cause mortality rate was 52.4% (95% CI, 43.3–62.1%) in patients with systolic blood pressure (SBP) <90 mmHg compared with 14.7% (95% CI, 13.3–16.2%) in normotensive patients.171 According to data from MAPPET, systemic hypotension, defined as SBP <90 mmHg or a reduction of at least 40 mmHg for at least 15 min, seems to carry a slightly lower risk compared with shock (in-hospital all-cause mortality, 15.2 vs. 24.5%, respectively).51 However, the expected mortality is still very high and justifies classification of a patient in the high-risk PE category, requiring immediate aggressive treatment.172
Syncope and cardiac arrest may occur in a patient with PE. In most cases, such an episode is related to persistent systemic hypotension and/or shock, which are markers of high risk. In the few patients who immediately regain consciousness and a stable blood pressure, risk assessment should be made on a case-by-case basis. It should take into account the severity of right ventricular dysfunction and the presence of impending embolism due to a floating right heart or proximal venous thrombi.
In summary, shock and hypotension are principal markers of high risk of early death in acute PE.
Markers of right ventricular dysfunction
Echocardiography
Echocardiographic findings suggesting RVD have been reported to occur in at least 25% of PE patients.173 A meta-analysis found more than a two-fold increased risk of PE-related mortality in patients with echocardiographic signs of right ventricular dysfunction.174 Two out of the seven studies included an estimation of risk in normotensive patients with PE.140,175 In such patients RVD had sensitivity of 56–61% and was related to the absolute increase in the early PE-related mortality of 4–5%.174 Importantly, patients with normal echocardiographic findings had an excellent outcome, with in hospital PE-related mortality <1% in most of the reported series.140–142 (Table 11).
Major trials reporting definitions and prognostic significance of RV dysfunction assessed by echocardiography in acute pulmonary embolism
| Author . | n . | Patient characteristics . | Echocardiographic criteria . | Early mortality . |
|---|---|---|---|---|
| . | . | . | . | RVD(+) vs. RVD(–) . |
| Goldhaber et al.175 | 101 | Normotensive | RV hypokinesis and dilatation | 4.3 vs. 0% |
| Ribeiro et al.141 | 126 | Normotensive and hypotensive | RVD | 12.8 vs. 0% |
| Kasper et al.142 | 317 | Normotensive and hypotensive | RV >30 mm or TI >2.8 m/s | 13 vs. 0.9% |
| Grifoni et al.140 | 162 | BP ≥100 mmHg | At least one of the following: | 4.6 vs. 0% |
| RV >30 mm or RV/LV >1 | ||||
| Paradox septal systolic motion | ||||
| AcT <90 ms or TIPG >30 mmHg | ||||
| Kucher et al.176 | 1035 | BP ≥90 mmHg | RVD | 16.3 vs. 9.4%a |
| Author . | n . | Patient characteristics . | Echocardiographic criteria . | Early mortality . |
|---|---|---|---|---|
| . | . | . | . | RVD(+) vs. RVD(–) . |
| Goldhaber et al.175 | 101 | Normotensive | RV hypokinesis and dilatation | 4.3 vs. 0% |
| Ribeiro et al.141 | 126 | Normotensive and hypotensive | RVD | 12.8 vs. 0% |
| Kasper et al.142 | 317 | Normotensive and hypotensive | RV >30 mm or TI >2.8 m/s | 13 vs. 0.9% |
| Grifoni et al.140 | 162 | BP ≥100 mmHg | At least one of the following: | 4.6 vs. 0% |
| RV >30 mm or RV/LV >1 | ||||
| Paradox septal systolic motion | ||||
| AcT <90 ms or TIPG >30 mmHg | ||||
| Kucher et al.176 | 1035 | BP ≥90 mmHg | RVD | 16.3 vs. 9.4%a |
All data refer to in-hospital PE-related mortality, except a30 day all-cause mortality.
RVD(+) = patients with RV dysfunction; RVD(–) = patients with normal RV function.
RV = right ventricle; BP = blood pressure; TI = tricuspid insufficiency; LV = left ventricle; AcT = acceleration time of right ventricular ejection; TIPG = tricuspid insufficiency peak gradient.
Major trials reporting definitions and prognostic significance of RV dysfunction assessed by echocardiography in acute pulmonary embolism
| Author . | n . | Patient characteristics . | Echocardiographic criteria . | Early mortality . |
|---|---|---|---|---|
| . | . | . | . | RVD(+) vs. RVD(–) . |
| Goldhaber et al.175 | 101 | Normotensive | RV hypokinesis and dilatation | 4.3 vs. 0% |
| Ribeiro et al.141 | 126 | Normotensive and hypotensive | RVD | 12.8 vs. 0% |
| Kasper et al.142 | 317 | Normotensive and hypotensive | RV >30 mm or TI >2.8 m/s | 13 vs. 0.9% |
| Grifoni et al.140 | 162 | BP ≥100 mmHg | At least one of the following: | 4.6 vs. 0% |
| RV >30 mm or RV/LV >1 | ||||
| Paradox septal systolic motion | ||||
| AcT <90 ms or TIPG >30 mmHg | ||||
| Kucher et al.176 | 1035 | BP ≥90 mmHg | RVD | 16.3 vs. 9.4%a |
| Author . | n . | Patient characteristics . | Echocardiographic criteria . | Early mortality . |
|---|---|---|---|---|
| . | . | . | . | RVD(+) vs. RVD(–) . |
| Goldhaber et al.175 | 101 | Normotensive | RV hypokinesis and dilatation | 4.3 vs. 0% |
| Ribeiro et al.141 | 126 | Normotensive and hypotensive | RVD | 12.8 vs. 0% |
| Kasper et al.142 | 317 | Normotensive and hypotensive | RV >30 mm or TI >2.8 m/s | 13 vs. 0.9% |
| Grifoni et al.140 | 162 | BP ≥100 mmHg | At least one of the following: | 4.6 vs. 0% |
| RV >30 mm or RV/LV >1 | ||||
| Paradox septal systolic motion | ||||
| AcT <90 ms or TIPG >30 mmHg | ||||
| Kucher et al.176 | 1035 | BP ≥90 mmHg | RVD | 16.3 vs. 9.4%a |
All data refer to in-hospital PE-related mortality, except a30 day all-cause mortality.
RVD(+) = patients with RV dysfunction; RVD(–) = patients with normal RV function.
RV = right ventricle; BP = blood pressure; TI = tricuspid insufficiency; LV = left ventricle; AcT = acceleration time of right ventricular ejection; TIPG = tricuspid insufficiency peak gradient.
Unfortunately, echocardiographic criteria of RVD differ among published studies and include RV dilatation, hypokinesis, increased RV/LV diameter ratio and increased velocity of the jet of tricuspid regurgitation.173,176 (Table 11). Thus, since a universal definition of RVD on echocardiography is lacking, only a completely normal result should be considered as defining low-risk PE. This is particularly important because in some of the trials echocardiographic signs of RV pressure overload alone (such as increased tricuspid insufficiency peak gradient and decreased acceleration time of right ventricular ejection) were considered sufficient to classify a patient to the RVD group.140 In addition to RVD, echocardiography can also identify two specific markers, each indicating doubled mortality risk in PE: right-to-left shunt through a patent foramen ovale and the presence of right heart thrombi.159,177
Computed tomography
Contrast-enhanced non-ECG-gated spiral CT used for pulmonary angiography allows assessment of the right-to-left ventricular dimension ratio but provides no direct information regarding RV function. With SDCT, identification of the longest minor axis of the RV and LV requires inspection of relevant transverse thoracic planes. An RV/LV ratio >1.0 was found in 58% of 120 initially stable patients with confirmed PE, and it had a PPV of 10% with regard to 30-day PE-related mortality (95% CI, 2.9–17.4%). The combination of RV/LV >1.0 and a CT-derived vascular obstruction index >40% increased the PPV for 3-month PE-related mortality to 18.8%. The predictive value of an RV/LV ratio ≤1.0 for an uneventful outcome was 100% (95% CI, 94.3–100%).178
Two studies by the same group reported experience with 16-detector CT. A pilot study found an RV/LV ratio >0.9, measured in the four-chamber view from reformatted, non-ECG-triggered images of the heart, to be slightly superior to measurements from axial views in identifying patients with PE and worse prognosis.179 In a follow-up study including 431 patients, RV/LV >0.9 was present in 64% of patients with PE, and its NPV and PPV for 30-day mortality were 92.3% and 15.6%, respectively (Web Site Table A). The hazard ratio of RV/LV >0.9 for predicting 30-day death was 5.17 (95% CI, 1.63–16.35; P = 0.005) after adjusting for other risk factors such as pneumonia, cancer, chronic obstructive pulmonary disease and age.180
When reports on smaller patient populations are also taken into consideration, most studies do suggest that CT scanning contributes to the risk stratification of patients with confirmed PE.181 Its greatest value appears to be the identification of low-risk patients based on the lack of RV dilatation (Web Site Table A). Other CT-derived indices, such as interventricular septum shape, or pulmonary artery dimensions, have not been found to be of prognostic relevance, while evidence regarding a more complex CT-derived vascular obstruction index is non-conclusive.182–184
Brain natriuretic peptide
Ventricular dysfunction is associated with increased myocardial stretch which leads to the release of brain natriuretic peptide (BNP). There is growing evidence that in acute PE levels of BNP or N-terminal proBNP (NT-proBNP) reflect the severity of RVD and haemodynamic compromise.185–188 Recent reports suggest that BNP or NT-proBNP as markers of RVD provide prognostic information additional to that derived from echocardiography.188,189
Although elevated BNP or NT-proBNP concentrations are related to worse outcome, their PPV is low (12–26%) (Web Site Table B). On the other hand, low levels of BNP or NT-proBNP can be reliably used for identification of patients with a good prognosis regarding short-term mortality or a complicated clinical outcome (NPV 94–100%).186,190–194
Other markers of RV dysfunction
Jugular vein distension, if not caused by cardiac tamponade or mediastinal tumours, may be a reliable sign of RVD in patients with PE. Other clinical signs, such as tricuspid regurgitation murmur and RV gallop, are more subjective and thus potentially misleading. New appearance of ECG signs of RV strain such as inversion of T waves in leads V1–V4, QR pattern in V1 lead, the classic S1Q3T3 pattern and incomplete or complete right bundle-branch block, are useful but of limited sensitivity.59,195–197 Right heart catheterization allows direct assessment of RV filling pressures and cardiac output, but its routine use for risk stratification in acute PE is not recommended.
In summary, RV dysfunction is related to intermediate risk of short-term mortality in acute PE. Prognostic assessment based on signs of RVD is limited by the lack of universally accepted criteria, which in some trials included isolated signs of pulmonary hypertension.
Markers of myocardial injury
Cardiac troponins
Transmural RV infarction despite patent coronary arteries has been found in autopsies of patients who died of massive PE.198,199 Several observational studies reported elevated cardiac troponin levels in PE.189,193,200–207 While RV myocardium might not necessarily be its only source, elevated plasma troponin levels have been repeatedly reported as associated with worse prognosis in patients with PE208 (Web Site Table C).
In an early study, the prevalence of a positive troponin T test, defined as >0.1 ng/mL, was reported in 0–35% and 50% of patients with non-massive, submassive and clinically massive PE, respectively.202 Positive troponin T was related to an in-hospital mortality of 44%, compared with 3% for negative troponin T [odds ratio (OR, 15.2; 95% CI, 1.2–190.4]. In another study, levels of troponins I and T correlated both with in-hospital mortality and a complicated clinical course.204 Increased in-hospital mortality has also been reported in normotensive patients with PE using cutoff values for troponin T as low as 0.01 ng/mL (OR, 21.0; 95% CI, 1.2–389.0)].206 Repeated blood sampling 6–12 h after admission should be considered, because initially negative results may convert to positive, with prognostic implications.206 A further study derived from a large therapeutic trial analysed the data of 458 consecutive patients with submassive PE and found that 13.5% of them had cardiac troponin I levels >0.5 ng/mL measured within 24 h of clinical presentation. Cardiac troponin elevation was associated with a 3.5-fold higher risk of all-cause death at three-month follow-up (95% CI, 1.0–11.9) (201). The prevalence of cTnI > 2.3 mg/L, corresponding to the levels indicating acute myocardial infarction, was 3.5% (95% CI, 2.0–5.6). Most trials reported PPV and NPV of elevated troponin for PE-related early mortality in the range of 12–44%, with very high NPV (99–100%), irrespective of various methods and cutoff values applied. A recent meta-analysis confirmed that elevated troponin levels were associated with increased mortality in the subgroup of haemodynamically stable patients (OR, 5.9; 95% CI, 2.7–12.9).208
New markers of myocardial injury
Few reports exist on the prognostic value of other biomarkers of myocardial injury in acute PE (Web Site Table C). Recently, heart-type fatty acid binding protein (H-FABP), an early marker of myocardial injury, was reported to be superior to troponin or myoglobin measurements for risk stratification of PE on admission. H-FABP >6 ng/mL had a PPV and NPV for early PE-related mortality of 23–37% and 96–100%, respectively.209,210
Combination of markers of myocardial injury and RV dysfunction
Simultaneous measurements of troponin and NT-proBNP were found to stratify normotensive patients with PE more accurately (Web Site Table D). PE-related 40-day mortality in the group with high levels of both cardiac troponin T and NT-proBNP exceeded 30%. Patients with an isolated elevation of NT-proBNP had an intermediate mortality rate (3.7%), while low levels of both biomarkers indicated a good short-term prognosis.189
An alternative approach consists of troponin testing combined with echocardiography. In one trial a combination of cardiac troponin I >0.1 ng/L and RV/LV >0.9 on echocardiography identified a subgroup with all-cause 30-day mortality of 38%.211 Preserved RV function without biochemical signs of myocardial injury identified patients with an excellent prognosis (Web Site Table E). 193,211,212
The currently available data do not allow the proposal of specific cutoff levels of markers that could be used for therapeutic decision-making in patients with non-high-risk PE. An ongoing multicentre randomized trial is evaluating the potential benefit of thrombolysis in normotensive patients with echocardiographic signs of RVD and abnormal troponin levels.
In summary, myocardial injury in patients with PE can be detected by troponin T or I testing. Positive results are related to an intermediate risk of short-term mortality in acute PE. Prognostic assessment based on signs of myocardial injury is limited by the lack of universally accepted criteria. New markers of injury and the concomitant assessment of markers of RVD may help improve the substratification of patients with acute PE.
Additional risk markers
Clinical and routine laboratory tests
Several variables collected during routine clinical and laboratory evaluation have prognostic significance in PE. Many of them are related to the pre-existing condition and the comorbidities of the individual patient rather than to the severity of the index PE episode. For example, in the ICOPER registry, age >70 years, cancer, congestive heart failure and chronic obstructive pulmonary disease were identified as prognostic factors.17 Several other clinical and laboratory features have been studied and risk scores for prognostic stratification have been proposed169,213 and validated.214,215 These risk scores use clinical variables and/or laboratory markers of prognosis. Some of them are intended to identify low-risk patients,169,214–216 who are potential candidates for early discharge and outpatient treatment, while other models seek to detect high-risk patients,193,206 who could benefit from more intensive management.
The Geneva prognostic score uses an eight-point scoring system and defines six predictors of adverse outcome: cancer and hypotension (<100 mmHg), 2 points each; heart failure, prior DVT, arterial hypoxaemia (PaO2 <8 kPa), and ultrasound-proven DVT, 1 point each.169 Male sex, tachycardia, hypothermia, altered mental status and low arterial oxygen saturation have also been identified as clinical prognostic markers and used in a clinical model of risk evaluation.213 In this risk score, 11 clinical variables are used to generate a score that divides patients into five risk classes for 30-day all-cause mortality, ranging from very low to very high risk (Table 12).
Routinely available clinical predictors of 30-day all-cause mortality in patients with acute PE
| Variable . | Points . |
|---|---|
| Age | 1/year |
| Male sex | 10 |
| Cancer | 30 |
| Heart failure | 10 |
| Chronic lung disease | 10 |
| Heart rate >110/min | 20 |
| Systolic blood pressure <100 mmHg | 30 |
| Respiratory rate ≥30/min | 20 |
| Body temperature <36°C | 20 |
| Disorientation, lethargy, stupor, coma | 60 |
| SaO2 <90% | 20 |
| Variable . | Points . |
|---|---|
| Age | 1/year |
| Male sex | 10 |
| Cancer | 30 |
| Heart failure | 10 |
| Chronic lung disease | 10 |
| Heart rate >110/min | 20 |
| Systolic blood pressure <100 mmHg | 30 |
| Respiratory rate ≥30/min | 20 |
| Body temperature <36°C | 20 |
| Disorientation, lethargy, stupor, coma | 60 |
| SaO2 <90% | 20 |
Data are from reference 214.
Risk categories (30-day all-cause mortality, %): class I, <65 points (0%); class II, 66–85 points (1%); class III, 86–105 points (3.1%); class IV, 106–125 points (10.4%); class, V >125 points (24.4%). Low risk = classes I and II (0–1%).
SaO2 = pulsoximetry.
Routinely available clinical predictors of 30-day all-cause mortality in patients with acute PE
| Variable . | Points . |
|---|---|
| Age | 1/year |
| Male sex | 10 |
| Cancer | 30 |
| Heart failure | 10 |
| Chronic lung disease | 10 |
| Heart rate >110/min | 20 |
| Systolic blood pressure <100 mmHg | 30 |
| Respiratory rate ≥30/min | 20 |
| Body temperature <36°C | 20 |
| Disorientation, lethargy, stupor, coma | 60 |
| SaO2 <90% | 20 |
| Variable . | Points . |
|---|---|
| Age | 1/year |
| Male sex | 10 |
| Cancer | 30 |
| Heart failure | 10 |
| Chronic lung disease | 10 |
| Heart rate >110/min | 20 |
| Systolic blood pressure <100 mmHg | 30 |
| Respiratory rate ≥30/min | 20 |
| Body temperature <36°C | 20 |
| Disorientation, lethargy, stupor, coma | 60 |
| SaO2 <90% | 20 |
Data are from reference 214.
Risk categories (30-day all-cause mortality, %): class I, <65 points (0%); class II, 66–85 points (1%); class III, 86–105 points (3.1%); class IV, 106–125 points (10.4%); class, V >125 points (24.4%). Low risk = classes I and II (0–1%).
SaO2 = pulsoximetry.
Elevated serum creatinine levels have also been reported as having significant prognostic relevance in acute PE patients.17,189 Another study found D-dimer levels below 1500 µg/L to have a 99% NPV in predicting all-cause 3-month mortality.217
In summary, multiple variables provided by clinical evaluation and routine laboratory tests are related to the prognosis in acute PE. Consideration of pre-existing patient-related factors may be useful in final risk stratification.
Strategy of prognostic assessment
Concurrently with the diagnosis of PE, prognostic assessment is required for risk stratification and therapeutic decision-making. Risk stratification of PE is performed in stages: it starts with clinical assessment of the haemodynamic status and continues with the help of laboratory tests (see Tables 4 and 5 in the subsection Severity of pulmonary embolism).
High-risk PE is diagnosed in the presence of shock or persistent arterial hypotension (defined as a systolic blood pressure <90 mmHg or a pressure drop of ≥40 mmHg for >15 min if not caused by new-onset arrhythmia, hypovolaemia or sepsis), and represents an immediately life-threatening emergency requiring specific management.33,171
In the remaining normotensive patients with non-high-risk PE, the presence of markers of RVD173 and/or myocardial injury208 identify intermediate-risk PE. It is likely that patients with intermediate-risk PE in whom markers of dysfunction and injury are both positive have a greater risk than patients with discordant results. Although short-term mortality above 30% has been reported, evidence is still insufficient to make a definitive statement.189,211
Haemodynamically stable patients without evidence of RVD or myocardial injury have low-risk PE. A patient with non-high-risk PE can be classified into the low-risk PE category if at least one of the myocardial dysfunction markers and at least one of the myocardial injury markers are assessed.
Routinely collected clinical and laboratory data may also have prognostic implications in acute PE when integrated into a weighted score (Table 12). Such a score, accounting also for the pre-existing condition and comorbidities of the patient, can be of help when considering early discharge and ambulatory treatment of patients with otherwise low-risk PE.
The anatomical distribution and burden of embolic occlusion of the pulmonary arterial bed can be assessed by means of angiography (Miller and Walsh scores),134,136 spiral CT (obstruction index)178 or lung scintigraphy.218 However, anatomical assessment seems less relevant for risk stratification than assessment based on functional (haemodynamic) consequences of PE, and is currently not recommended for prognostic purposes.
In summary, evaluation of haemodynamic status, signs of RVD and myocardial injury and the assessment of additional patient-related factors are useful for optimal risk stratification.
| Recommendations: prognostic assessment . | Classa . | Levelb . |
|---|---|---|
| • Initial risk stratification of suspected and/or confirmed PE based on the presence of shock and hypotension is recommended to distinguish between patients with high and non-high-risk of PE-related early mortality | I | B |
| • In non-high-risk PE patients, further stratification to an intermediate- or low-risk PE subgroup based on the presence of imaging or biochemical markers of RVD and myocardial injury should be considered | IIa | B |
| Recommendations: prognostic assessment . | Classa . | Levelb . |
|---|---|---|
| • Initial risk stratification of suspected and/or confirmed PE based on the presence of shock and hypotension is recommended to distinguish between patients with high and non-high-risk of PE-related early mortality | I | B |
| • In non-high-risk PE patients, further stratification to an intermediate- or low-risk PE subgroup based on the presence of imaging or biochemical markers of RVD and myocardial injury should be considered | IIa | B |
aClass of recommendation.
bLevel of evidence.
Treatment
Haemodynamic and respiratory support
Acute RV failure with resulting low systemic output is the leading cause of death in patients with high-risk PE. Therefore, supportive treatment is of vital importance in patients with PE and RV failure.
Experimental studies indicate that aggressive volume expansion may worsen RV function by causing mechanical overstretch and/or by reflex mechanisms that depress contractility.219 On the other hand, a small clinical study observed an increase in cardiac index from 1.6 to 2.0 L/min/m2 after a 500 ml dextran infusion in normotensive patients with acute PE and low cardiac index.220 It appears that a modest fluid challenge may help increase cardiac index in patients with PE, low cardiac index and normal blood pressure.
Isoproterenol is an inotropic drug which also induces pulmonary vasodilatation, but these favourable effects are often outweighed by peripheral vasodilatation. The resulting hypotension may lead to decreased RV perfusion and ischaemia.221 Norepinephrine appears to improve RV function via a direct positive inotropic effect while also improving RV coronary perfusion by peripheral vascular alpha receptor stimulation and the increase in systemic blood pressure. No clinical data are available on the effects of norepinephrine in PE, and its use should probably be limited to hypotensive patients.222 In a small series of patients requiring admission to an intensive care unit for PE, dobutamine raised cardiac output and improved oxygen transport and tissue oxygenation at a constant arterial PO2.223 In another study of 10 patients with PE, low cardiac index and normal blood pressure, a 35% increase in cardiac index was observed under intravenous dobutamine infusion at a moderate dosage without significant change in heart rate, systemic arterial pressure or mean pulmonary arterial pressure.224 Accordingly, the use of dobutamine and/or dopamine can be considered for patients with PE, low cardiac index and normal blood pressure. However, raising the cardiac index above physiological values may aggravate the ventilation–perfusion mismatch by further redistributing flow from (partly) obstructed to non-obstructed vessels.221,223 Epinephrine combines the beneficial properties of norepinephrine and dobutamine without the systemic vasodilatory effects of the latter drug.221 In patients with PE and shock, epinephrine may exert beneficial effects.225
Vasodilators decrease pulmonary arterial pressure and pulmonary vascular resistance in animals and, to a lesser extent, in patients with PE.40,42 The main concern is the lack of specificity of these drugs for the pulmonary vasculature after systemic (intravenous) administration. To overcome this limitation, vasodilators may be administered by inhalation.226 According to data from small clinical studies, inhalation of nitric oxide may improve the haemodynamic status and gas exchange in patients with PE.227–229 There are few data with respect to inhaled aerosolized prostacyclin in the treatment of pulmonary hypertension secondary to PE.226,230,231
Preliminary experimental data suggest that levosimendan may restore right ventricular–pulmonary arterial coupling in acute PE as a result of combined pulmonary vasodilation and increased RV contractility.232
There is increasing interest in the use of endothelin antagonists and phosphodiesterase-5 inhibitors in PE. In experimental studies, antagonism of endothelin receptors attenuated the severity of pulmonary hypertension caused by massive PE.233,234 Sildenafil infusion also attenuated the increase in pulmonary artery pressure in experimental PE.235,236
Hypoxaemia and hypocapnia are frequently encountered in patients with PE, although they are of moderate severity in most cases. A patent foramen ovale may aggravate hypoxaemia due to shunting when the right atrial pressure exceeds the left atrial pressure.177,237 Hypoxaemia is usually reversed with nasal oxygen, and mechanical ventilation is rarely necessary. Oxygen consumption should be minimized with measures to reduce fever and agitation, and by instituting mechanical ventilation if the work of breathing is excessive. When mechanical ventilation is required, care should be taken to limit its adverse haemodynamic effects. In particular, positive intrathoracic pressure induced by mechanical ventilation may reduce venous return and worsen RV failure in patients with massive PE. Therefore, positive end-expiratory pressure should be applied with caution. Low tidal volumes (approximately 6 ml/kg lean body weight) should be used in an attempt to keep the end-inspiratory plateau pressure below 30 cm H2O.238
In summary, haemodynamic and respiratory support is necessary in patients with suspected or confirmed PE presenting with shock or hypotension.
Thrombolysis
Randomized trials175,218,239–244 have consistently shown that thrombolytic therapy rapidly resolves thromboembolic obstruction and exerts beneficial effects on haemodynamic parameters. In an early small trial, an 80% increase in cardiac index and a 40% decrease in pulmonary arterial pressure was observed after 72 h of streptokinase treatment.245 In the Plasminogen Activator Italian Multicenter Study 2, serial angiograms revealed that 100 mg of recombinant tissue plasminogen activator (rtPA) induced a 12% decrease in vascular obstruction at the end of the 2 h infusion period, whereas no change was observed in patients receiving heparin.239 The effect of rtPA was associated with a 30% reduction in mean pulmonary arterial pressure and a 15% increase in cardiac index. One of the largest thrombolysis trials demonstrated a significant reduction in mean RV end-diastolic area on echocardiography 3 h after treatment with rtPA.175
With regard to the comparison of different thrombolytic agents, the Urokinase–Streptokinase Pulmonary Embolism Trial (USPET) documented equal efficacy of urokinase and streptokinase infused over a period of 12–24 h.246 In more recent randomized trials,247,248100 mg rtPA infused over 2 h led to faster angiographic and haemodynamic improvement compared with urokinase infused over 12 or 24 h at the rate of 4400 IU/kg/h, although the results no longer differed at the end of the urokinase infusion. Similarly, the 2 h infusion of rtPA appeared to be superior to a 12 h streptokinase infusion (at 100 000 IU/h), but no difference was observed when the same streptokinase dose was given over 2 h.249,250 Furthermore, two trials that compared the 2 h, 100 mg rtPA regimen with a short infusion (over 15 min) of 0.6 mg/kg rtPA reported non-significant trends for both slightly faster improvements and slightly higher bleeding rates with the 2 h regimen.251,252 Direct local infusion of rtPA via a catheter in the pulmonary artery (at a reduced dosage) was not found to offer any advantages over systemic intravenous thrombolysis.253 This approach should generally be avoided, as it also carries an increased risk of bleeding at the puncture site.
The approved thrombolytic regimens of streptokinase, urokinase and rtPA are shown in Table 13. Satisfactory haemodynamic results also have been obtained with double-bolus reteplase, two injections (10 U) 30 min apart.254 Preliminary uncontrolled data appear to support the efficacy and safety of tenecteplase in acute PE.255 Heparin should not be infused concurrently with streptokinase or urokinase, but it can be given during alteplase administration.
Approved thrombolytic regimens for pulmonary embolism
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h |
| Accelerated regimen: 1.5 million IU over 2 h | |
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h |
| Accelerated regimen: 3 million IU over 2 h | |
| rtPA | 100 mg over 2 h |
| or 0.6 mg/kg over 15 min (maximum dose 50 mg) |
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h |
| Accelerated regimen: 1.5 million IU over 2 h | |
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h |
| Accelerated regimen: 3 million IU over 2 h | |
| rtPA | 100 mg over 2 h |
| or 0.6 mg/kg over 15 min (maximum dose 50 mg) |
rtPA = recombinant tissue plasminogen activator.
Approved thrombolytic regimens for pulmonary embolism
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h |
| Accelerated regimen: 1.5 million IU over 2 h | |
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h |
| Accelerated regimen: 3 million IU over 2 h | |
| rtPA | 100 mg over 2 h |
| or 0.6 mg/kg over 15 min (maximum dose 50 mg) |
| Streptokinase | 250 000 IU as a loading dose over 30 min, followed by 100 000 IU/h over 12–24 h |
| Accelerated regimen: 1.5 million IU over 2 h | |
| Urokinase | 4400 IU/kg as a loading dose over 10 min, followed by 4400 IU/kg/h over 12–24 h |
| Accelerated regimen: 3 million IU over 2 h | |
| rtPA | 100 mg over 2 h |
| or 0.6 mg/kg over 15 min (maximum dose 50 mg) |
rtPA = recombinant tissue plasminogen activator.
Overall, approximately 92% of patients can be classified as responders to thrombolysis based on clinical and echocardiographic improvement within the first 36 h.256 The greatest benefit is observed when treatment is initiated within 48 h of symptom onset,243 but thrombolysis can still be useful in patients who have had symptoms for 6–14 days.257
Although of rapid onset, the haemodynamic benefits of thrombolysis over heparin appear to be confined to the first few days. One week after treatment, the changes in the severity of vascular obstruction218,239 and the reversal of RVD258 were no longer different between thrombolysis-treated and heparin-treated patients.
Thrombolytic therapy carries a significant risk of bleeding, especially when predisposing conditions or comorbidities exist. Summarized data from randomized trials218,239,241,247,248,252,253,259–261 reveal a 13% cumulative rate of major bleeding and a 1.8% rate of intracranial/fatal haemorrhage. In the most recent of these trials,175,259 life-threatening haemorrhage has been less common. This appears to be in line with the observation that thrombolysis-related bleeding rates are lower when non-invasive imaging methods are used to confirm PE,262 a strategy that has been adopted increasingly over the past 10 years.
The overall effects of thrombolysis on the clinical outcome of patients with PE are difficult to assess. With one exception,259 thrombolysis trials have not been designed to address clinical endpoints. In weighing the risk of bleeding against the possible clinical benefits of thrombolysis, it is important to keep in mind the natural history and prognosis of high-risk, intermediate-risk and low-risk PE. Hence, contraindications to thrombolysis that are considered absolute in acute myocardial infarction, e.g. surgery within the preceding 3 weeks or gastrointestinal bleeding within the last month (Table 14) might become relative in a patient with immediately life-threatening, high-risk PE.
Contraindications to fibrinolytic therapy
| Absolute contraindicationsa |
| • Haemorrhagic stroke or stroke of unknown origin at any time |
| • Ischaemic stroke in preceding 6 months |
| • Central nervous system damage or neoplasms |
| • Recent major trauma/surgery/head injury (within preceding 3 weeks) |
| • Gastrointestinal bleeding within the last month |
| • Known bleeding |
| Relative contraindications |
| • Transient ischaemic attack in preceding 6 months |
| • Oral anticoagulant therapy |
| • Pregnancy or within 1 week post partum |
| • Non-compressible punctures |
| • Traumatic resuscitation |
| • Refractory hypertension (systolic blood pressure >180 mmHg) |
| • Advanced liver disease |
| • Infective endocarditis |
| • Active peptic ulcer |
| Absolute contraindicationsa |
| • Haemorrhagic stroke or stroke of unknown origin at any time |
| • Ischaemic stroke in preceding 6 months |
| • Central nervous system damage or neoplasms |
| • Recent major trauma/surgery/head injury (within preceding 3 weeks) |
| • Gastrointestinal bleeding within the last month |
| • Known bleeding |
| Relative contraindications |
| • Transient ischaemic attack in preceding 6 months |
| • Oral anticoagulant therapy |
| • Pregnancy or within 1 week post partum |
| • Non-compressible punctures |
| • Traumatic resuscitation |
| • Refractory hypertension (systolic blood pressure >180 mmHg) |
| • Advanced liver disease |
| • Infective endocarditis |
| • Active peptic ulcer |
From reference 263.
aContraindications to thrombolysis that are considered absolute, e.g. in acute myocardial infarction, might become relative in a patient with immediately life-threatening high-risk PE.
Contraindications to fibrinolytic therapy
| Absolute contraindicationsa |
| • Haemorrhagic stroke or stroke of unknown origin at any time |
| • Ischaemic stroke in preceding 6 months |
| • Central nervous system damage or neoplasms |
| • Recent major trauma/surgery/head injury (within preceding 3 weeks) |
| • Gastrointestinal bleeding within the last month |
| • Known bleeding |
| Relative contraindications |
| • Transient ischaemic attack in preceding 6 months |
| • Oral anticoagulant therapy |
| • Pregnancy or within 1 week post partum |
| • Non-compressible punctures |
| • Traumatic resuscitation |
| • Refractory hypertension (systolic blood pressure >180 mmHg) |
| • Advanced liver disease |
| • Infective endocarditis |
| • Active peptic ulcer |
| Absolute contraindicationsa |
| • Haemorrhagic stroke or stroke of unknown origin at any time |
| • Ischaemic stroke in preceding 6 months |
| • Central nervous system damage or neoplasms |
| • Recent major trauma/surgery/head injury (within preceding 3 weeks) |
| • Gastrointestinal bleeding within the last month |
| • Known bleeding |
| Relative contraindications |
| • Transient ischaemic attack in preceding 6 months |
| • Oral anticoagulant therapy |
| • Pregnancy or within 1 week post partum |
| • Non-compressible punctures |
| • Traumatic resuscitation |
| • Refractory hypertension (systolic blood pressure >180 mmHg) |
| • Advanced liver disease |
| • Infective endocarditis |
| • Active peptic ulcer |
From reference 263.
aContraindications to thrombolysis that are considered absolute, e.g. in acute myocardial infarction, might become relative in a patient with immediately life-threatening high-risk PE.
In summary, thrombolytic therapy is the first-line treatment in patients with high-risk PE presenting with cardiogenic shock and/or persistent arterial hypotension, with very few absolute contraindications. Routine use of thrombolysis in non-high-risk patients is not recommended, but may be considered in selected patients with intermediate-risk PE and after thorough consideration of conditions increasing the risk of bleeding. Thrombolytic therapy should be not used in patients with low-risk PE.
Surgical pulmonary embolectomy
Several decades before the introduction of medical treatment for PE, the first successful surgical pulmonary embolectomy was performed in 1924.264 For a long time, pulmonary embolectomy remained a rare rescue operation and there were few data on its efficacy and safety. Recently however, interdisciplinary therapeutic approaches to PE involving the cardiac surgeon have begun to emerge in several centres.265,266
Traditionally, pulmonary embolectomy has been reserved for patients with PE who may necessitate cardiopulmonary resuscitation. It is also performed in patients with contraindications or inadequate response to thrombolysis, and in those with patent foramen ovale and intracardiac thrombi.256,265 Transportable extracorporeal assist systems with percutaneous femoral cannulation can be helpful in critical situations, providing circulation and oxygenation and thus time for definitive diagnosis.267–269 In one series, pulmonary embolectomy was also performed in patients with PE and RVD without persistent hypotension or shock.270
In centres with routine cardiac surgery programmes, pulmonary embolectomy is a simple operation. Following rapid induction of anaesthesia and median sternotomy, normothermic cardiopulmonary bypass is instituted. Unless intracardiac thrombi or a patent foramen ovale are present, aortic crossclamping and cardioplegic cardiac arrest should be avoided.266,270 With an incision of the PA trunk and usually an additional arteriotomy of the right pulmnary artery, clots can be removed from both pulmonary arteries using blunt grasping instruments under direct vision. Prolonged periods of postoperative cardiopulmonary bypass and weaning may be necessary until the recovery of RV function. Bleeding may be a problem in patients with preoperative thrombolysis, although previous thrombolysis is not a contraindication to surgical embolectomy.270 The routine perioperative placement of an inferior vena caval filter remains controversial.
In the past, the results of pulmonary embolectomy were considered poor as early mortality rates were high.271–273 With a broader spectrum of indications for embolectomy in patients with RVD but in the absence of severe shock, early mortality rates of 6–8% have been reported.256,266,270
Patients presenting with an episode of acute PE superimposed on a history of long-lasting dyspnoea and severe pulmonary hypertension are likely to suffer from chronic thromboembolic pulmonary hypertension. These patients are not candidates for embolectomy as they need specific pulmonary endarterectomy, which should be performed in specialized centres.274
In summary, with current surgical techniques pulmonary embolectomy is a valuable therapeutic option in patients with high-risk PE in whom thrombolysis is absolutely contraindicated or has failed.
Percutaneous catheter embolectomy and fragmentation
Percutanous techniques to open a partially occluded pulmonary trunk or major pulmonary arteries may be life-saving in some critical situations of high-risk PE.275,276 Although the available evidence is limited to case reports or series, such procedures can be performed as an alternative to thrombolysis when there are absolute contraindications, as adjunctive therapy when thrombolysis has failed to improve haemodynamics, or as an alternative to surgery if immediate access to cardiopulmonary bypass is unavailable.
The Greenfield suction embolectomy catheter was introduced in 1969277 and it remains the only device with FDA approval. Fragmentation and dispersion using conventional cardiac catheters275 or specially designed pulmonary catheters with rotational or other macerating devices278 has evolved technically since the late 1980s. Variably good results are described with currently used devices, but these have never been rigorously evaluated in clinical trials.
Deployment of some of the devices (which can be introduced via catheter sheaths ranging from 6 to 11 F) within the pulmonary arteries may require dexterity, particularly if the right main pulmonary artery is occluded. Catheter techniques should only be used in the main arteries since fragmentation within the smaller branches is unlikely to be of benefit and may damage the more delicate structures, with risk of perforation.279
Haemodynamic improvement can be dramatic following successful thrombus fragmentation. Crucially, the procedure should be terminated as soon as haemodynamics improve, regardless of the angiographic result. Substantial improvement in pulmonary blood flow may result from what appears to be only modest angiographic change.
Complications of percutaneous procedures include local damage to the puncture site, usually the femoral vein, perforation of cardiac structures, tamponade and contrast reactions. Iliac and caval flow can be assessed angiographically, but obstruction by remaining thrombus is rarely a problem.
In summary, catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in high-risk PE patients when thrombolysis is absolutely contraindicated or has failed.
Initial anticoagulation
Anticoagulant treatment plays a pivotal role in the management of patients with PE. The need for immediate anticoagulation in patients with PE is based on a landmark study which was performed in the 1960s and demonstrated the benefits of unfractionated heparin in comparison with no treatment.280 The objectives of the initial anticoagulant treatment of PE are to prevent death and recurrent events with an acceptable rate of bleeding complications.
Rapid anticoagulation can only be achieved with parenteral anticoagulants, such as intravenous unfractionated heparin, subcutaneous low-molecular-weight heparin (LMWH) or subcutaneous fondaparinux.281 Considering the high mortality rate in untreated patients, anticoagulant treatment should be considered in patients with suspected PE while awaiting definitive diagnostic confirmation.
Treatment with parenteral anticoagulants is usually followed by the administration of oral vitamin K antagonists (VKAs). The requirement for an initial course of heparin in addition to VKAs, compared with starting treatment with VKA therapy alone, was established in a randomized controlled study that reported a three-fold higher rate of recurrent VTE in patients who received VKAs only.282 If intravenous unfractionated heparin is given, a weight-adjusted regimen of 80 U/kg as a bolus injection followed by infusion at the rate of 18 U/kg/h should be preferred to fixed dosages of heparin.283 Subsequent doses of unfractionated heparin should be adjusted using an activated partial thromboplastin time (aPTT)-based nomogram to rapidly reach and maintain aPTT prolongation (between 1.5 and 2.5 times control) corresponding to therapeutic heparin levels (Table 15). The aPTT should be measured 4–6 h after the bolus injection and then 3 h after each dose adjustment, or once daily when the target therapeutic dose has been reached.
Adjustment of intravenous unfractionated heparin dosage based on the activated partial thromboplastin time
| Activated partial thromboplastin time . | Change of dosage . |
|---|---|
| <35 s (<1.2 times control) | 80 U/kg bolus; increase infusion rate by 4 U/kg/h |
| 35–45 s (1.2–1.5 times control) | 40 U/kg bolus; increase infusion rate by 2 U/kg/h |
| 46–70 s (1.5–2.3 times control) | No change |
| 71–90 s (2.3–3.0 times control) | Reduce infusion rate by 2 U/kg/h |
| >90 s (>3.0 times control) | Stop infusion for 1 h, then reduce infusion rate by 3 U/kg/h |
| Activated partial thromboplastin time . | Change of dosage . |
|---|---|
| <35 s (<1.2 times control) | 80 U/kg bolus; increase infusion rate by 4 U/kg/h |
| 35–45 s (1.2–1.5 times control) | 40 U/kg bolus; increase infusion rate by 2 U/kg/h |
| 46–70 s (1.5–2.3 times control) | No change |
| 71–90 s (2.3–3.0 times control) | Reduce infusion rate by 2 U/kg/h |
| >90 s (>3.0 times control) | Stop infusion for 1 h, then reduce infusion rate by 3 U/kg/h |
Data are from reference 283. This article was published in Arch Intern Med, Vol. 156, Raschke RA, Gollihare B, Peirce JC. The effectiveness of implementing the weight-based heparin nomogram as a practice guideline, 1645–1649. Copyright © (1996) American Medical Association. All Rights reserved.
Adjustment of intravenous unfractionated heparin dosage based on the activated partial thromboplastin time
| Activated partial thromboplastin time . | Change of dosage . |
|---|---|
| <35 s (<1.2 times control) | 80 U/kg bolus; increase infusion rate by 4 U/kg/h |
| 35–45 s (1.2–1.5 times control) | 40 U/kg bolus; increase infusion rate by 2 U/kg/h |
| 46–70 s (1.5–2.3 times control) | No change |
| 71–90 s (2.3–3.0 times control) | Reduce infusion rate by 2 U/kg/h |
| >90 s (>3.0 times control) | Stop infusion for 1 h, then reduce infusion rate by 3 U/kg/h |
| Activated partial thromboplastin time . | Change of dosage . |
|---|---|
| <35 s (<1.2 times control) | 80 U/kg bolus; increase infusion rate by 4 U/kg/h |
| 35–45 s (1.2–1.5 times control) | 40 U/kg bolus; increase infusion rate by 2 U/kg/h |
| 46–70 s (1.5–2.3 times control) | No change |
| 71–90 s (2.3–3.0 times control) | Reduce infusion rate by 2 U/kg/h |
| >90 s (>3.0 times control) | Stop infusion for 1 h, then reduce infusion rate by 3 U/kg/h |
Data are from reference 283. This article was published in Arch Intern Med, Vol. 156, Raschke RA, Gollihare B, Peirce JC. The effectiveness of implementing the weight-based heparin nomogram as a practice guideline, 1645–1649. Copyright © (1996) American Medical Association. All Rights reserved.
It should be noted that aPTT is not a perfect marker of the intensity of the anticoagulant effect of heparin. Therefore, it is not necessary to increase the infusion rate above 1667 U/h (corresponding to 40 000 U/day) provided the anti-factor Xa heparin level is at least 0.35 IU/mL, even if the aPTT ratio is below the therapeutic range.284
Low molecular weight heparins should be given with care in patients with renal failure and their dose adjusted according to anti-Xa level. Intravenous unfractionated heparin should be the preferred mode of initial anticoagulation for patients with severe renal impairment (creatinine clearance <30 ml/min), as it is not eliminated by the kidneys, and for those at high risk of bleeding, as its anticoagulant effect can be rapidly reversed. For all other cases of acute PE, unfractionated heparin can be replaced by LMWH given subcutaneously at weight-adjusted doses without monitoring.
Several trials compared the efficacy and safety of subcutaneous LMWH with those of unfractionated heparin. Major studies285–293 with a total of 1951 patients with non-high-risk symptomatic PE or with asymptomatic PE in association with symptomatic DVT were included in a meta-analysis294 At the end of the study treatment (5–14 days), LMWH was at least as efficacious as unfractionated heparin regarding the rate of recurrent VTE (OR, 0.63; 95% CI, 0.33–1.18) and at least as safe regarding major bleeding (OR, 0.67; 95% CI, 0.36–1.27). All-cause mortality was similar in the two groups (OR, 1.20; 95% CI, 0.59–2.45).
Table 16 lists the low molecular weight heparins that are currently approved for the treatment of acute PE. Other LMWH, approved for the treatment of DVT, are sometimes also used in PE. LMWH cannot be recommended for high-risk PE with haemodynamic instability, as such patients were excluded from randomized trials testing the efficacy and safety of these drugs in PE. Anti-factor Xa activity (anti-Xa) levels need not be measured routinely in a patient receiving LMWH, but they should be considered in patients with severe renal failure as well as during pregnancy.295 The usual time to take samples for the anti-Xa assay is 4 h after the morning injection, when anti-Xa levels are highest. A target range of 0.6–1.0 IU/mL is suggested for twice-daily administration, and a target range of 1.0–2.0 IU/mL is suggested for once-daily administration, although neither recommendation is firmly founded.295
Subcutaneous regimens of low molecular-weight heparins and fondaparinux approved for the treatment of pulmonary embolism
| . | Dose . | Interval . |
|---|---|---|
| Enoxaparin | 1.0 mg/kg | Every 12 h |
| or 1.5 mg/kga | Once dailya | |
| Tinzaparin | 175 U/kg | Once daily |
| Fondaparinux | 5 mg (body weight <50 kg) | Once daily |
| 7.5 mg (body weight 50–100 kg) | ||
| 10 mg (body weight >100 kg) |
| . | Dose . | Interval . |
|---|---|---|
| Enoxaparin | 1.0 mg/kg | Every 12 h |
| or 1.5 mg/kga | Once dailya | |
| Tinzaparin | 175 U/kg | Once daily |
| Fondaparinux | 5 mg (body weight <50 kg) | Once daily |
| 7.5 mg (body weight 50–100 kg) | ||
| 10 mg (body weight >100 kg) |
In patients with cancer, Dalteparin is approved for extended treatment of symptomatic VTE (proximal DVT and/or PE), at an initial dose of 200 U/kg s.c. once daily (see drug labelling for details).
aOnce-daily injection of enoxaparin at the dose of 1.5 mg/kg is approved for inpatient (hospital) treatment of PE in the United States and in some, but not all, European countries.
Subcutaneous regimens of low molecular-weight heparins and fondaparinux approved for the treatment of pulmonary embolism
| . | Dose . | Interval . |
|---|---|---|
| Enoxaparin | 1.0 mg/kg | Every 12 h |
| or 1.5 mg/kga | Once dailya | |
| Tinzaparin | 175 U/kg | Once daily |
| Fondaparinux | 5 mg (body weight <50 kg) | Once daily |
| 7.5 mg (body weight 50–100 kg) | ||
| 10 mg (body weight >100 kg) |
| . | Dose . | Interval . |
|---|---|---|
| Enoxaparin | 1.0 mg/kg | Every 12 h |
| or 1.5 mg/kga | Once dailya | |
| Tinzaparin | 175 U/kg | Once daily |
| Fondaparinux | 5 mg (body weight <50 kg) | Once daily |
| 7.5 mg (body weight 50–100 kg) | ||
| 10 mg (body weight >100 kg) |
In patients with cancer, Dalteparin is approved for extended treatment of symptomatic VTE (proximal DVT and/or PE), at an initial dose of 200 U/kg s.c. once daily (see drug labelling for details).
aOnce-daily injection of enoxaparin at the dose of 1.5 mg/kg is approved for inpatient (hospital) treatment of PE in the United States and in some, but not all, European countries.
Because of the risk of heparin-induced thrombocytopenia (HIT), monitoring of the platelet count is necessary during treatment with unfractionated or low-molecular-weight heparin (see Specific problems).
The selective factor Xa inhibitor fondaparinux given subcutaneously at weight-adjusted doses without monitoring is a valuable alternative to LMWH. Because of its half-life of 15–20 h, fondaparinux allows once-a-day subcutaneous administration (Table 16). An open-label trial which enrolled 2213 patients with acute PE and no indication for thrombolytic therapy found that weight-adjusted, fixed-dose, fondaparinux was associated with rates of recurrent VTE (3.8 vs. 5.0% at 3 months) and major bleeding (1.3 vs. 1.1%) similar to those obtained with intravenous unfractionated heparin.296 As no proven HIT case has ever been observed with fondaparinux, platelet count monitoring is not needed with this compound. Fondaparinux is contraindicated in severe renal failure with creatinine clearance <20 ml/min.
Anticoagulation with unfractionated heparin, LMWH or fondaparinux should be continued for at least 5 days. Two randomized clinical trials in patients with proximal DVT reported that unfractionated heparin given for 5–7 days is as effective as unfractionated heparin given for 10–14 days, provided that it is followed by adequate long-term anticoagulant therapy.297,298 VKAs should be initiated as soon as possible and preferably on the same day as the initial anticoagulant. Parenteral anticoagulants should be stopped when the international normalized ratio (INR) lies between 2.0 and 3.0 for at least 2 consecutive days. If warfarin is used, a starting dose of 5 or 7.5 mg is preferred over higher doses. Two trials performed in hospitalized patients showed that starting warfarin at a dose of 5 mg was associated with less excessive anticoagulation compared with 10 mg. Taken together, these data suggest that warfarin can usually be started at a dose of 10 mg in younger (e.g. <60 years), otherwise healthy outpatients, and at a dose of 5 mg in older patients and in those who are hospitalized. Subsequent doses should be adjusted to maintain the INR at a target of 2.5 (range 2.0–3.0).
There is no evidence concerning the benefit of immobilization for the clinical outcome of patients with pulmonary embolism. Indeed, most of the data are related to patients with DVT. In these patients, recent studies have shown a similar incidence of new PE on routine repeat lung scanning with early ambulation and leg compression compared with immobilization.299–301 A recent Cochrane review that combined the findings of the most recent studies estimated that wearing stockings markedly reduced the cumulative incidence of post-thrombotic syndrome in patients with proximal DVT 2 years after the index event (OR, 0.3; 95% CI, 0.2–0.5).302
Recent studies have explored the possibility of outpatient (home) treatment for patients with PE, but none of them specifically randomized patients with acute PE to be treated either in hospital or at home. It is conceivable that this approach could be reserved for selected patients with low-risk PE.
Rapid-acting oral anticoagulants could replace parenteral agents for the initial VTE treatment. A number of new oral anticoagulants, particularly Xa and IIa inhibitors not requiring monitoring, are currently under clinical evaluation.
In summary, anticoagulation with unfractionated heparin, LMWH or fondaparinux should be initiated without delay in patients with confirmed PE and those with a high or intermediate clinical probability of PE while the diagnostic workup is still ongoing. Except for patients at high risk of bleeding and those with severe renal dysfunction, subcutaneous LMWH or fondaparinux rather then intravenous unfractionated heparin should be considered for initial treatment.
Therapeutic strategies
High-risk pulmonary embolism
Patients with PE presenting with shock or hypotension (previously considered ‘clinically massive’ PE) are at high risk of in-hospital death, particularly during the first few hours after admission.303 Intravenous unfractionated heparin should be the preferred mode of initial anticoagulation in these patients, as LMWH and fondaparinux have not been tested in the setting of hypotension and shock. To date, only one small randomized trial has specifically addressed the benefits of thrombolysis (streptokinase) vs. heparin in high-risk PE.199 Pooled data from five trials that included patients with high-risk PE appear to suggest a significant reduction in death or PE recurrence after thrombolysis (Table 17).139 Therefore, thrombolysis should be undertaken in patients with high-risk PE unless there are absolute contraindications to its use. Uncontrolled data also suggest that thrombolysis may be a safe and effective alternative to surgery in patients with PE and free-floating thrombi in the right heart.304,305
Meta-analysis of thrombolysis trials in patients with pulmonary embolism
| Outcome . | Trials that included patients with massive PE . | Trials that excluded patients with massive PE . | ||||
|---|---|---|---|---|---|---|
| . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . |
| Recurrent PE or death | 12/128 (9.4%) | 24/126 (19.0%) | 0.45 (0.22–0.92) | 13/246 (5.3%) | 12/248 (4.8%) | 1.07 (0.50–2.30) |
| Recurrent PE | 5/128 (3.9%) | 9/126 (7.1%) | 0.61 (0.23–1.62) | 5/246 (2.0%) | 7/248 (2.8%) | 0.76 (0.28–2.08) |
| Death | 8/128 (6.2%) | 16/126 (12.7%) | 0.47 (0.20–1.10) | 8/246 (3.3%) | 6/248 (2.4%) | 1.16 (0.44–3.05) |
| Major bleeding | 28/128 (21.9%) | 15/126 (11.9%) | 1.98 (1.00–3.92) | 6/246 (2.4%) | 8/248 (3.2%) | 0.67 (0.24–1.86) |
| Outcome . | Trials that included patients with massive PE . | Trials that excluded patients with massive PE . | ||||
|---|---|---|---|---|---|---|
| . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . |
| Recurrent PE or death | 12/128 (9.4%) | 24/126 (19.0%) | 0.45 (0.22–0.92) | 13/246 (5.3%) | 12/248 (4.8%) | 1.07 (0.50–2.30) |
| Recurrent PE | 5/128 (3.9%) | 9/126 (7.1%) | 0.61 (0.23–1.62) | 5/246 (2.0%) | 7/248 (2.8%) | 0.76 (0.28–2.08) |
| Death | 8/128 (6.2%) | 16/126 (12.7%) | 0.47 (0.20–1.10) | 8/246 (3.3%) | 6/248 (2.4%) | 1.16 (0.44–3.05) |
| Major bleeding | 28/128 (21.9%) | 15/126 (11.9%) | 1.98 (1.00–3.92) | 6/246 (2.4%) | 8/248 (3.2%) | 0.67 (0.24–1.86) |
Adapted from reference 139. This article was published in Circulation, Vol. 110, Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials, 744–749. © (2004) American Heart Association, Inc.
n = number of patients with study endpoint; N = total number of patients; OR = odds ratio.
Meta-analysis of thrombolysis trials in patients with pulmonary embolism
| Outcome . | Trials that included patients with massive PE . | Trials that excluded patients with massive PE . | ||||
|---|---|---|---|---|---|---|
| . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . |
| Recurrent PE or death | 12/128 (9.4%) | 24/126 (19.0%) | 0.45 (0.22–0.92) | 13/246 (5.3%) | 12/248 (4.8%) | 1.07 (0.50–2.30) |
| Recurrent PE | 5/128 (3.9%) | 9/126 (7.1%) | 0.61 (0.23–1.62) | 5/246 (2.0%) | 7/248 (2.8%) | 0.76 (0.28–2.08) |
| Death | 8/128 (6.2%) | 16/126 (12.7%) | 0.47 (0.20–1.10) | 8/246 (3.3%) | 6/248 (2.4%) | 1.16 (0.44–3.05) |
| Major bleeding | 28/128 (21.9%) | 15/126 (11.9%) | 1.98 (1.00–3.92) | 6/246 (2.4%) | 8/248 (3.2%) | 0.67 (0.24–1.86) |
| Outcome . | Trials that included patients with massive PE . | Trials that excluded patients with massive PE . | ||||
|---|---|---|---|---|---|---|
| . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . | Thrombolysis (n/N) . | Heparin (n/N) . | Odds ratio (95% CI) . |
| Recurrent PE or death | 12/128 (9.4%) | 24/126 (19.0%) | 0.45 (0.22–0.92) | 13/246 (5.3%) | 12/248 (4.8%) | 1.07 (0.50–2.30) |
| Recurrent PE | 5/128 (3.9%) | 9/126 (7.1%) | 0.61 (0.23–1.62) | 5/246 (2.0%) | 7/248 (2.8%) | 0.76 (0.28–2.08) |
| Death | 8/128 (6.2%) | 16/126 (12.7%) | 0.47 (0.20–1.10) | 8/246 (3.3%) | 6/248 (2.4%) | 1.16 (0.44–3.05) |
| Major bleeding | 28/128 (21.9%) | 15/126 (11.9%) | 1.98 (1.00–3.92) | 6/246 (2.4%) | 8/248 (3.2%) | 0.67 (0.24–1.86) |
Adapted from reference 139. This article was published in Circulation, Vol. 110, Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials, 744–749. © (2004) American Heart Association, Inc.
n = number of patients with study endpoint; N = total number of patients; OR = odds ratio.
In patients with absolute contraindications to thrombolysis and in those in whom thrombolysis has failed to improve haemodynamic status, surgical embolectomy is the preferred therapy. If this is not immediately available, catheter embolectomy or thrombus fragmentation may be considered, though the safety and efficacy of such interventions has not been adequately documented.
Non-high-risk pulmonary embolism
Normotensive patients with non-high-risk PE generally have a favourable short-term prognosis. For most cases of acute non-high-risk PE without severe renal dysfunction, LMWH or fondaparinux, given subcutaneously at weight-adjusted doses without monitoring, is the treatment of choice. Pooled data from six trials revealed no clinical benefits from thrombolytic therapy in this group (Table 17).139
Intermediate-risk pulmonary embolism defines patients who appear haemodynamically stable on admission but have evidence of RVD and/or myocardial injury. A recent trial randomized 256 patients with intermediate-risk PE and no relative contraindications to thrombolysis (Table 14) to heparin vs. rtPA treatment.259 The primary combined endpoint, in-hospital death or clinical deterioration requiring escalation of treatment, was significantly reduced in the thrombolysis group compared with the heparin group. The difference was due to a more frequent need for secondary (emergency) thrombolysis in the heparin group during the hospital stay, while the overall mortality rate was not affected by thrombolysis. Thus, it appears that the risk/benefit ratio of thrombolysis may be favourable in selected patients with intermediate-risk PE, particularly in those without an elevated risk of bleeding (Table 14). A large multinational European trial has been initiated and will attempt to resolve the controversy still surrounding the appropriate treatment of this patient group.
Low-risk pulmonary embolism defines patients without principal PE-related risk factors, who can be considered for early discharge, if proper outpatient care and anticoagulant treatment can be provided. Pre-existing, non-specific patient-related risk factors, as well as the risk of bleeding, should always be considered.
| Recommendations: acute treatment . | Classa . | Levelb . |
|---|---|---|
| High-risk pulmonary embolism | ||
| • Anticoagulation with unfractionated heparin should be initiated without delay in patients with high-risk PE | I | A |
| • Systemic hypotension should be corrected to prevent progression of RV failure and death due to PE | I | C |
| • Vasopressive drugs are recommended for hypotensive patients with PE | I | C |
| • Dobutamine and dopamine may be used in patients with PE, low cardiac output and normal blood pressure | IIa | B |
| • Aggressive fluid challenge is not recommended | III | B |
| • Oxygen should be administered in patients with hypoxaemia | I | C |
| • Thrombolytic therapy should be used in patients with high-risk PE presenting with cardiogenic shock and/or persistent arterial hypotension | I | A |
| • Surgical pulmonary embolectomy is a recommended therapeutic alternative in patients with high-risk PE in whom thrombolysis is absolutely contraindicated or has failed | I | C |
| • Catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in high-risk patients when thrombolysis is absolutely contraindicated or has failed | IIb | C |
| Non-high-risk pulmonary embolism | ||
| • Anticoagulation should be initiated without delay in patients with high or intermediate clinical probability of PE while diagnostic workup is still ongoing | I | C |
| • Use of LMWH or fondaparinux is the recommended form of initial treatment for most patients with non-high-risk PE | I | A |
| • In patients at high risk of bleeding and in those with severe renal dysfunction, unfractionated heparin with an aPTT target range of 1.5–2.5 times normal is a recommended form of initial treatment | I | C |
| • Initial treatment with unfractionated heparin, LMWH or fondaparinux should be continued for at least 5 days and | I | A |
| may be replaced by vitamin K antagonists only after achieving target INR levels for at least 2 consecutive days | I | C |
| • Routine use of thrombolysis in non–high-risk PE patients is not recommended, but it may be considered in selected patients with intermediate-risk PE | IIb | B |
| • Thrombolytic therapy should be not used in patients with low-risk PE | III | B |
| Recommendations: acute treatment . | Classa . | Levelb . |
|---|---|---|
| High-risk pulmonary embolism | ||
| • Anticoagulation with unfractionated heparin should be initiated without delay in patients with high-risk PE | I | A |
| • Systemic hypotension should be corrected to prevent progression of RV failure and death due to PE | I | C |
| • Vasopressive drugs are recommended for hypotensive patients with PE | I | C |
| • Dobutamine and dopamine may be used in patients with PE, low cardiac output and normal blood pressure | IIa | B |
| • Aggressive fluid challenge is not recommended | III | B |
| • Oxygen should be administered in patients with hypoxaemia | I | C |
| • Thrombolytic therapy should be used in patients with high-risk PE presenting with cardiogenic shock and/or persistent arterial hypotension | I | A |
| • Surgical pulmonary embolectomy is a recommended therapeutic alternative in patients with high-risk PE in whom thrombolysis is absolutely contraindicated or has failed | I | C |
| • Catheter embolectomy or fragmentation of proximal pulmonary arterial clots may be considered as an alternative to surgical treatment in high-risk patients when thrombolysis is absolutely contraindicated or has failed | IIb | C |
| Non-high-risk pulmonary embolism | ||
| • Anticoagulation should be initiated without delay in patients with high or intermediate clinical probability of PE while diagnostic workup is still ongoing | I | C |
| • Use of LMWH or fondaparinux is the recommended form of initial treatment for most patients with non-high-risk PE | I | A |
| • In patients at high risk of bleeding and in those with severe renal dysfunction, unfractionated heparin with an aPTT target range of 1.5–2.5 times normal is a recommended form of initial treatment | I | C |
| • Initial treatment with unfractionated heparin, LMWH or fondaparinux should be continued for at least 5 days and | I | A |
| may be replaced by vitamin K antagonists only after achieving target INR levels for at least 2 consecutive days | I | C |
| • Routine use of thrombolysis in non–high-risk PE patients is not recommended, but it may be considered in selected patients with intermediate-risk PE | IIb | B |
| • Thrombolytic therapy should be not used in patients with low-risk PE | III | B |
aClass of recommendation.
bLevel of evidence.
Long-term anticoagulation and secondary prophylaxis
The long-term anticoagulant treatment of patients with PE is aimed at preventing fatal and non-fatal recurrent VTE events. VKAs are used in the vast majority of patients, while LMWH may be an effective and safe alternative to VKAs in cancer patients.306,307 VKAs should be given at doses adjusted to maintain a target INR of 2.5 (range 2.0–3.0).
Most of the studies focusing on long-term anticoagulation for VTE included patients with DVT, and only one study specifically focused on patients with PE.308 However, the implications for treatment of proximal DVT or PE are very similar, the main difference being that recurrent episodes are about three times more likely to be PE after an initial PE than after an initial DVT.10
The need for long-term anticoagulant treatment of VTE is supported by three lines of evidence, all from randomized trials. One of these studies showed a 20% rate of symptomatic extension and/or recurrence within 3 months in patients with symptomatic calf-vein thrombosis not receiving long-term anticoagulant treatment.309 Another study proved the lack of efficacy of low-dose unfractionated heparin as an alternative to VKAs after proximal DVT.310 In further studies, reducing the duration of treatment to 4 or 6 weeks resulted in an increased recurrence rate compared with the conventional duration of 3–6 months.311,312
Clinical trials that have evaluated different durations of anticoagulant therapy can be divided into three categories according to the duration of therapy compared: (i) short vs. intermediate duration; (ii) different intermediate durations of therapy; and (iii) indefinite vs. intermediate duration. The main findings from these studies are: (i) the duration of anticoagulant therapy should not be limited to 4–6 weeks in patients with unprovoked VTE; (ii) a similar risk of recurrence is expected if anticoagulants are stopped after 6 or 12 months compared with 3 months; (iii) indefinite treatment reduces the risk of recurrent VTE by about 90%, but this advantage is partially offset by the risk of major bleeding.38,311,313,314 In general, VKAs are highly effective in preventing recurrent VTE during treatment, but they do not eliminate the risk of subsequent recurrence after treatment discontinuation.38,314 Thus, the duration of anticoagulant treatment in a particular patient represents a balance between the estimated risk of recurrence after treatment discontinuation and the risk of bleeding complications while on treatment. An additional factor may be the inconvenience of treatment with VKAs in patients with INR 2–3, including the need for regular laboratory monitoring.
Active cancer is a major risk factor for recurrence of VTE, the rate of recurrence being about 20% during the first 12 months after the index event.315,316 As a risk factor for recurrence, cancer outweighs all other patient-related risks. Therefore, cancer patients are candidates for indefinite anticoagulant treatment after a first episode of PE. In a randomized study of patients with DVT and cancer, the LMWH dalteparin, given at the dose of 200 U/kg once daily for 4–6 weeks followed by 75% of the initial dose given once daily for up to 6 months, was more effective than warfarin in preventing recurrent VTE.317 Accordingly, at least 6 months of treatment with LMWH are recommended for patients with VTE and cancer, followed by treatment with LMWH or VKAs as long as the disease is considered active.306
With the exception of cancer patients, the risk of recurrent VTE after treatment discontinuation is related to the features of the index VTE event. A study that followed patients with a first episode of acute PE found that the recurrence rate after treatment discontinuation was approximately 2.5% per year after PE associated with reversible risk factors compared with 4.5% per year after idiopathic (unprovoked) PE.308 Similar observations were made in other prospective studies on patients with DVT.311 Reversible risk factors for VTE include surgery, trauma, medical illness, oestrogen therapy and pregnancy. For patients with PE secondary to a transient (reversible) risk factor, treatment with a VKA for 3 months should be preferred over shorter periods, with the possible exception of patients with distal DVT associated with a reversible risk factor. Treatment for longer than 3 months is generally not recommended, provided that the causative transient risk factor has been removed.
Risk stratification of patients with unprovoked PE is more complex and remains an unresolved issue. The following risk factors may help identify patients at higher long-term risk (relative risk 1.5–2.0) of VTE recurrence: (i) one or more previous episodes of VTE; (ii) antiphospholipid antibody syndrome; (iii) hereditary thrombophilia; (iv) male vs. female sex; and (v) residual thrombosis in the proximal veins. An additional risk factor for VTE recurrence in patients with PE appears to be persistence of RVD at hospital discharge as assessed by echocardiography.318 On the other hand, a negative D-dimer test 1 month after withdrawal of the VKA seems to be a protective factor for VTE recurrence (relative risk 0.4).319
Among carriers of molecular thrombophilia, patients with lupus anticoagulant, those with confirmed deficit of protein C or protein S, and patients homozygous for factor V Leiden or homozygous for PTG20210A may be candidates for indefinite anticoagulant treatment after a first unprovoked VTE. No evidence of a clinical benefit of extended anticoagulant treatment is currently available for heterozygous carriers of factor V Leiden or the prothrombin mutation G20210A.
In addition to the risk of recurrence, the risk of bleeding needs to be considered in determining the duration of treatment. Among the risk factors for major bleeding during anticoagulant therapy, the following appear to be of clinical relevance: (i) old age, particularly above 75 years; (ii) previous gastrointestinal bleeding, particularly if not associated with a reversible cause; (iii) previous non-cardioembolic stroke; chronic renal or hepatic disease; (iv) concomitant antiplatelet therapy (to be avoided if possible); (v) other serious acute or chronic illness; (vi) poor anticoagulant control; and (vii) suboptimal monitoring of anticoagulant therapy.
Based on the above considerations, patients with unprovoked PE should be treated with VKA for at least 3 months. All patients should then be evaluated for the risks vs. benefits of indefinite therapy. Indefinite anticoagulant therapy is recommended for patients with a first unprovoked proximal DVT or PE and a low risk of bleeding, when this is consistent with the patient's preference. Indefinite treatment is recommended for most patients with a second unprovoked DVT or PE.
Reduced VKA doses for extended treatment in patients with idiopathic VTE were shown to be effective and safe when compared with placebo,320 but they were less effective and not safer when compared with conventional intensity anticoagulation.321 This approach should not be generalized, but reserved for selected cases.
The efficacies of different durations of chronic anticoagulant treatment in preventing the development of chronic thromboembolic pulmonary hypertension are unknown.
An oral anticoagulant with no need for laboratory monitoring and dose adjustment is currently needed for the long-term treatment of PE. At least two types of oral agents, the selective thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban and apixaban, are currently under investigation for the long-term treatment of PE.
| Recommendations: long-term treatment . | Classa . | Levelb . |
|---|---|---|
| • For patients with PE secondary to a transient (reversible) risk factor, treatment with a VKA is recommended for 3 months | I | A |
| • For patients with unprovoked PE, treatment with a VKA is recommended for at least 3 months | I | A |
| • Patients with a first episode of unprovoked PE and low risk of bleeding, and in whom stable anticoagulation can be achieved, may be considered for long-term oral anticoagulation | IIb | B |
| • For patients with a second episode of unprovoked PE, long-term treatment is recommended | I | A |
| • In patients who receive long-term anticoagulant treatment, the risk/benefit ratio of continuing such treatment should be reassessed at regular intervals | I | C |
| • For patients with PE and cancer, LMWH should be considered for the first 3–6 months … | IIa | B |
| after this period, anticoagulant therapy with VKA or LMWH should be continued indefinitely or until the cancer is considered cured | I | C |
| • In patients with PE, the dose of VKA should be adjusted to maintain a target INR of 2.5 (range 2.0–3.0) regardless of treatment duration | I | A |
| Recommendations: long-term treatment . | Classa . | Levelb . |
|---|---|---|
| • For patients with PE secondary to a transient (reversible) risk factor, treatment with a VKA is recommended for 3 months | I | A |
| • For patients with unprovoked PE, treatment with a VKA is recommended for at least 3 months | I | A |
| • Patients with a first episode of unprovoked PE and low risk of bleeding, and in whom stable anticoagulation can be achieved, may be considered for long-term oral anticoagulation | IIb | B |
| • For patients with a second episode of unprovoked PE, long-term treatment is recommended | I | A |
| • In patients who receive long-term anticoagulant treatment, the risk/benefit ratio of continuing such treatment should be reassessed at regular intervals | I | C |
| • For patients with PE and cancer, LMWH should be considered for the first 3–6 months … | IIa | B |
| after this period, anticoagulant therapy with VKA or LMWH should be continued indefinitely or until the cancer is considered cured | I | C |
| • In patients with PE, the dose of VKA should be adjusted to maintain a target INR of 2.5 (range 2.0–3.0) regardless of treatment duration | I | A |
aClass of recommendation.
bLevel of evidence.
Venous filters
Interruption of the inferior vena cava as a method or preventing PE was first suggested by Trousseau in 1868. Venous filters became available in the late 1960s and percutaneous deployment was made possible almost 30 years ago.322 Filters are usually placed in the infrarenal portion of the inferior vena cava (IVC). If thrombus is identified in the IVC below the renal veins, more superior placement may be indicated.
Permanent IVC filters may provide lifelong protection against PE; however, they are associated with complications and late sequelae, including recurrent DVT episodes and development of the post-thrombotic syndrome.
Complications of permanent IVC filters are common, although they are infrequently fatal.323 Early complications, including insertion site thrombosis, occur in 10% of patients. Late complications are much more frequent and include recurrent DVT in approximately 20% and the post-thrombotic syndrome in 40% of patients. Overall, occlusion of the vena cava affects approximately 22% of patients at 5 years and 33% at 9 years, regardless of the use and duration of anticoagulation.324–326 Other IVC filters are designed to be retrieved after their period of required usage has passed. It is recommended that retrievable devices should be removed within 2 weeks of implantation. However, available data indicate that temporary devices are often left in situ for longer periods of time, with a late complication rate of up to 10%, including migration and device thrombosis.327 The exact risk/benefit ratio of IVC filters is difficult to determine because follow-up has been incomplete in most series and reported recurrence did not require objective tests for PE. In the only randomized study to date, 400 patients with DVT (with or without PE) were treated either with an anticoagulant (unfractionated vs. low molecular weight heparin plus an oral anticoagulant) alone, or with an anticoagulant combined with the insertion of a vena cava filter. During the first 12 days, the PE rate was 1.1% with the filter in place vs. 4.8% with anticoagulant alone (P = 0.03). However, during the 2-year follow-up, the difference became non-significant. Although there was no difference in total mortality at 12 days (2.5% in each group), four of five deaths in the non-filter group were due to PE vs. none of five deaths in the filter group.291 Overall, this trial, now with 8 years of follow-up data available,324 shows a reduced risk of recurrent PE at the cost of an increased risk of recurrent DVT with no overall effect on survival in patients undergoing permanent IVC filter insertion.
At present, the systematic use of venous filters is not recommended in the general population with VTE. On the other hand, venous filters may be used when there are absolute contraindications to anticoagulation and a high risk of VTE recurrence, including, for example, the period immediately after neurosurgery or other major surgery. They may also be considered in pregnant women who develop extensive thrombosis in the weeks before delivery. As soon as it is safe to use anticoagulants, retrievable filters should be removed; however, there are no data from prospective randomized trials to dictate optimal duration of IVC filter use.
There are no data to support the routine use of venous filters in patients with free-floating proximal deep venous thrombosis. In one series, the PE recurrence rate among such patients who received adequate anticoagulant treatment alone was low (3.3%).328 Similarly, planned thrombolysis is not an indication for prophylactic filter insertion.
| Recommendations: venous filters . | Classa . | Levelb . |
|---|---|---|
| • IVC filters may be used when there are absolute contraindications to anticoagulation and a high risk of VTE recurrence | IIb | B |
| • The routine use of IVC filters in patients with PE is not recommended | III | B |
| Recommendations: venous filters . | Classa . | Levelb . |
|---|---|---|
| • IVC filters may be used when there are absolute contraindications to anticoagulation and a high risk of VTE recurrence | IIb | B |
| • The routine use of IVC filters in patients with PE is not recommended | III | B |
a Class of recommendation.
b Level of evidence.
IVC, inferior vena cava; VTE, venous thromboembolism.
Specific problems
Pregnancy
The incidence of PE during pregnancy ranges between 0.3 and 1 per 1000 deliveries.329 PE is the leading cause of pregnancy-related maternal death in developed countries.330 The risk of PE is higher in the post-partum period, particularly after a Caesarean section. The clinical features of PE are no different in pregnancy compared with the non-pregnant state.331 However, pregnant women often present with breathlessness, and this symptom should be interpreted with caution, especially when isolated and neither severe nor of acute onset. PaO2 is normal during pregnancy. However, arterial blood should be drawn in the upright position as the PaO2 may be lower in the supine position during the third trimester.332
Diagnosis of pulmonary embolism in pregnancy
Exposure of the fetus to ionizing radiation is a concern when investigating suspected PE during pregnancy. However, this concern is largely overcome by the hazards of missing a potentially fatal diagnosis. Moreover, erroneously assigning a diagnosis of PE to a pregnant woman is also fraught with risk since it unnecessarily exposes the fetus and mother to the risk of anticoagulant treatment. Therefore, investigations should aim for diagnostic certainty.
Plasma D-dimer levels increase physiologically throughout pregnancy. In a prospective study, however, around 50% of women had a normal D-dimer level at the 20th week of pregnancy.85 A normal D-dimer value has the same exclusion value for PE in pregnant women as in other patients with suspected PE. Therefore, it should be measured even though the probability of a negative result is lower than in other patients with suspected PE, in order to avoid unnecessary exposure of the fetus to X-rays. An elevated D-dimer result should be followed by lower limb CUS since a positive result warrants anticoagulation treatment and makes thoracic imaging unnecessary. If ultrasonography is negative, however, the diagnosis should be pursued.
The amount of radiation absorbed by the fetus in different diagnostic tests is shown in Table 18. The upper limit with regard to the danger of injury to the fetus is considered to be 50 mSv (50 000 µGy)333 and all radiological tests fall well below this limit. Recent data on chest CT suggest that the radiation dose delivered to the fetus is lower than that of perfusion lung scintigraphy in the first or second trimester334 and that it can therefore be performed safely. However, perfusion lung scintigraphy is also a reasonable option; its diagnostic yield is high in pregnant women (75%) and a retrospective series reported excellent outcomes in pregnant women left untreated based on a normal perfusion scan.331 Perfusion scanning compares favourably with CT as far as exposure of breast tissue to radiation is concerned. Ventilation phase does not appear to add enough information to warrant the additional radiation. In women left undiagnosed by perfusion lung scintigraphy, however, CT should be preferred over pulmonary angiography, which carries a significantly higher X-ray exposure for the fetus (2.2–3.7 mSv).333
Estimated radiation absorbed by fetus in procedures for diagnosing pulmonary embolism
| Test . | Estimated radiation . | |
|---|---|---|
| . | μGy . | mSv . |
| Chest radiography | <10 | 0.01 |
| Perfusion lung scan with technetium 99m-labelled albumin (1–2 mCi) | 60–120 | 0.06–012 |
| Ventilation lung scan | 200 | 0.2 |
| CT angiography | ||
| First trimester | 3–20 | 0.003–0.02 |
| Second trimester | 8–77 | 0.008–0.08 |
| Third trimester | 51–130 | 0.051–0.13 |
| Pulmonary angiography by femoral access | 2210–3740 | 2.2–3.7 |
| Pulmonary angiography by brachial access | <500 | <0.5 |
| Test . | Estimated radiation . | |
|---|---|---|
| . | μGy . | mSv . |
| Chest radiography | <10 | 0.01 |
| Perfusion lung scan with technetium 99m-labelled albumin (1–2 mCi) | 60–120 | 0.06–012 |
| Ventilation lung scan | 200 | 0.2 |
| CT angiography | ||
| First trimester | 3–20 | 0.003–0.02 |
| Second trimester | 8–77 | 0.008–0.08 |
| Third trimester | 51–130 | 0.051–0.13 |
| Pulmonary angiography by femoral access | 2210–3740 | 2.2–3.7 |
| Pulmonary angiography by brachial access | <500 | <0.5 |
Data are from references 333 and 334.
Estimated radiation absorbed by fetus in procedures for diagnosing pulmonary embolism
| Test . | Estimated radiation . | |
|---|---|---|
| . | μGy . | mSv . |
| Chest radiography | <10 | 0.01 |
| Perfusion lung scan with technetium 99m-labelled albumin (1–2 mCi) | 60–120 | 0.06–012 |
| Ventilation lung scan | 200 | 0.2 |
| CT angiography | ||
| First trimester | 3–20 | 0.003–0.02 |
| Second trimester | 8–77 | 0.008–0.08 |
| Third trimester | 51–130 | 0.051–0.13 |
| Pulmonary angiography by femoral access | 2210–3740 | 2.2–3.7 |
| Pulmonary angiography by brachial access | <500 | <0.5 |
| Test . | Estimated radiation . | |
|---|---|---|
| . | μGy . | mSv . |
| Chest radiography | <10 | 0.01 |
| Perfusion lung scan with technetium 99m-labelled albumin (1–2 mCi) | 60–120 | 0.06–012 |
| Ventilation lung scan | 200 | 0.2 |
| CT angiography | ||
| First trimester | 3–20 | 0.003–0.02 |
| Second trimester | 8–77 | 0.008–0.08 |
| Third trimester | 51–130 | 0.051–0.13 |
| Pulmonary angiography by femoral access | 2210–3740 | 2.2–3.7 |
| Pulmonary angiography by brachial access | <500 | <0.5 |
Data are from references 333 and 334.
Treatment of pulmonary embolism in pregnancy
The treatment of PE in pregnancy is based mainly on heparin—either unfractionated heparin or LWMH, neither of which crosses the placenta or is found in breast milk in any significant amount. Increasing experience suggests that LMWH is safe in pregnancy335,336 and its use is endorsed by several reports.337,338 As there are no specific data in the setting of pregnancy, treatment should consist of a weight-adjusted dose of LMWH. Adaptation according to anti-Xa monitoring may be considered in women at extremes of body weight or with renal disease, or whenever felt necessary. The heparin treatment should be given throughout the entire pregnancy. As there are no data in pregnancy, fondaparinux cannot be used in this situation. VKA antagonists cross the placenta and are associated with a well-defined embryopathy during the first trimester.339 Administration of VKA antagonists in the third trimester can result in fetal and neonatal haemorrhage as well as in placental abruption. Warfarin may be associated with central nervous system anomalies in any trimester in pregnancy. Although some experts recommend the cautious use of warfarin during the second trimester of pregnancy by analogy with a frequently used regimen in pregnant women with mechanical heart valves,340 this therapeutic approach should be avoided whenever possible. The management of labour and delivery require particular attention. Epidural analgesia cannot be used unless LMWH is discontinued at least 12 h before an epidural approach. Treatment can be resumed 12–24 h after withdrawal of the epidural catheter. In any case, close collaboration between obstetrician, anaesthetist and attending physician is recommended.
After delivery, heparin treatment may be replaced by anticoagulation with VKA. Anticoagulant treatment should be administered for at least 3 months after delivery. VKAs can be given even to breast-feeding mothers.
There is published information on 36 women treated with thrombolytic agents in pregnancy, massive PE being the indication in about one-third of them.341 Streptokinase was the agent most frequently used. Streptokinase (and probably other thrombolytic drugs) does not cross the placenta. However in mothers the overall incidence of bleeding is about 8%, usually from the genital tract. This risk does not seem unreasonable compared with the death rate seen in patients with massive PE treated with heparin alone. At the time of delivery, thrombolytic treatment should not be used except in extremely severe cases and if surgical embolectomy is not immediately available. Indications for cava filters in pregnant women are similar to those in other patients with PE.
In summary, in pregnant women with a clinical suspicion of PE an accurate diagnosis is necessary, because a prolonged course of heparin is required. All diagnostic modalities, including CT scanning, may be used without significant risk to the fetus. Low molecular weight heparins are recommended in confirmed PE; VKAs are not recommended during the first and third trimesters and may be considered with caution in the second trimester of pregnancy.
Anticoagulant treatment should be administered for at least 3 months after delivery.
Malignancy
The association of PE and cancer is well documented. Cohort studies and clinical trials both suggest that patients presenting with an idiopathic or unprovoked PE subsequently develop a cancer in about 10% of the cases over a 5–10 year follow-up period.342–344
The risk of thrombosis among cancer patients is about four times higher than in the general population and the risk increases to about 6.7-fold in patients receiving chemotherapy.345 A number of anticancer agents, as well as of drugs used in supportive cancer therapy, have been associated with an increased risk of venous thromboembolic events. The combination of hormonal and chemotherapy seems to play a synergistic role in the development of thrombosis in patients with cancer.346 The use of antiangiogenic agents such as thalidomide is also frequently complicated by thrombosis.347,348
Cancer patients with VTE are more likely to develop recurrent thromboembolic complications and major bleeding during anticoagulant treatment than those without malignancy.315,316 These risks correlate with the extent of cancer.
The use of more or less sophisticated imaging techniques, such as ultrasound, endoscopic gastrointestinal examinations, CT scanning, magnetic resonance imaging and nuclear medicine examinations for routine screening of cancer in patients with so-called idiopathic PE, is still controversial despite extensive investigations.76,82,349,350 Most authors suggest that an extensive workup should be performed only if there is a strong suspicion of cancer after a careful clinical history and physical examination, routine blood tests and chest X-ray.351–353
The association between cancer and activation of blood coagulation has been known since Trousseau's time. The hypercoagulable state often encountered in cancer patients not only acts as an important risk factor for thrombosis, but may also play a role in tumour progression and metastasis. Heparins and other anticoagulants have been reported as having some anticancer effects.354,355 The results of a randomized trial307 pointing to positive effects of LMWH in tumour biology gave further encouragement to this concept, which is still under active investigation.
Several papers have been published regarding the efficacy advantages of LMWH relative to coumarin derivatives. In the CLOT (Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent VTE in Patients With Cancer) trial,306 the use of dalteparin relative to oral anticoagulants was associated with improved survival in patients with solid tumours who did not have metastatic disease at the time of an acute venous thromboembolic event. In the FAMOUS (Fragmin Advanced Malignancy Outcome Study) trial,307 this benefit in survival was found only in a subgroup of patients with a better prognosis but not in patients with advanced cancer. All studies seem to indicate that there is a good safety profile for the administration of LMWH to cancer patients, resulting in the suggestion that these agents seem to be safer than VKAs in this context. For patients with PE and cancer, LMWH should be considered for the first 3–6 months. After this period, anticoagulant therapy with VKAs or LMWH should be continued indefinitely, or until the cancer is considered cured.
In summary, malignancy is a major predisposing factor for the development and recurrence of VTE. However, routine extensive screening for cancer in patients with a first episode of non-provoked PE is not recommended. In cancer patients with confirmed PE, LMWH should be considered for the first 3–6 months of treatment and anticoagulant treatment should be continued indefinitely or until definitive cure of the cancer.
Right heart thrombi
In patients with PE, it is not uncommon to see right heart thrombi at echocardiography. Patients with right heart thrombi have lower systemic blood pressure, higher prevalence of hypotension, higher heart rate, and more frequently RV hypokinesis at echocardiography in comparison with other patients with PE.157,159 This unfavourable association explains the relatively high prevalence of right heart thrombi (7–18%) in PE patients admitted to intensive care units.156,305,356 The prevalence of right heart thrombi in unselected patients with PE is below 4% and probably would not warrant routine echocardiography screening in clinically stable patients.159
In patients with PE, the presence of right heart thrombi, especially those that are mobile, probably in transit from the peripheral veins to the lungs, is associated with increased early mortality.159,304,305,357 Whether right heart thrombi are an independent risk factor for mortality is unclear. However, the available data indicate that the presence of mobile right heart thrombi should be considered as a potentially life-threatening condition associated with a high risk of recurrent PE. In patients with mobile right heart thrombi, the death rate has been reported to be as high as 80–100% when left untreated.304,358 In these patients, the treatment of choice is controversial. In the ICOPER registry, thrombolytic treatment was the preferred option but the 14-day mortality was above 20%.159 In contrast, excellent results of such treatment were reported in a recent series of 16 patients, in which 50, 75 and 100% of clots disappeared from the right heart within first 2, 12 and 24 h after administration of thrombolysis, respectively.157 All patients survived 30 days even though the disappearance of thrombi seemed to have resulted from their embolization to pulmonary circulation rather than to in situ lysis. However, publication bias should also be considered, and current evidence does not allow us assess survival rates with thrombolytic treatment compared with surgery in individual patients.
Heparin used alone seems to be insufficient even in patients whose clinical condition otherwise would appear benign.159,304,357 Surgical or catheter embolectomy remain as alternatives, but data are scarce. Surgical embolectomy seems a treatment of choice in cases of right heart thrombi straddling the interatrial septum through the foramen ovale,359 though good outcomes with medical treatment have also been reported.359,360
Whichever therapy is selected, it should be implemented without delay: in the presence of unequivocal echocardiographic visualization of a mobile right heart thrombus no further diagnostic tests are needed.
In summary, right heart thrombi, particularly when mobile, i.e. in transit from the systemic veins, are associated with a significantly increased risk of early mortality in patients with acute PE. Immediate therapy is necessary, but optimal treatment is controversial in the absence of controlled trials. Thrombolysis and embolectomy are probably both effective whereas anticoagulation alone appears less effective.
Heparin-induced thrombocytopenia
This is a potentially serious complication of heparin therapy. The immune-mediated type of HIT is referred to as type II to distinguish it from other non-immune-mediated and more benign forms. It is caused by immunoglobulin G directed against the platelet factor 4–heparin complex.361,362 HIT type II usually occurs between 5 and 14 days after exposure to heparin, or earlier in cases of re-exposure. Paradoxically, despite a moderate to severe fall in the platelet count, patients with HIT are at high risk of venous and arterial thromboembolic events.
Several factors may influence the frequency of HIT: heparin type (unfractionated heparin > LMWH > fondaparinux); patient type (surgical > medical); and sex (female > male). The incidence of HIT ranges from 1 to 3% in patients exposed to unfractionated heparin and is about 1% in patients receiving LMWH. However, a recent meta-analysis did not confirm a lower prevalence of HIT among patients with VTE treated with LMWH compared with unfractionated heparin.363 HIT type II occurs in about 2% of patients undergoing heart or thoracic surgery requiring cardiopulmonary bypass.361,364
HIT type II should be suspected in all patients with a previous normal platelet count who present a fall to less than 100 000/mm3 or to less than 50% of the basal value. The diagnosis of HIT type II should always be confirmed by excluding other causes of thrombocytopenia and by performing specific immunological tests.362
If there is a clinical suspicion of HIT type II, heparin should be discontinued and the patient should be switched to an alternative agent, if anticoagulation is still required, until the platelet count returns above 100 000/mm3. Direct thrombin inhibitors, such as lepirudin and argatroban, are effective agents in treating complications of HIT.365 Isolated oral anticoagulation is contraindicated in the acute phase of this disorder but can be considered as a long-term treatment of the thromboembolic events.
No formally proven case of HIT has been reported with fondaparinux,366 which has been anecdotally reported as being used in the management of HIT type II.
In summary, HIT is a life-threatening immunological complication of heparin therapy. Monitoring of platelet counts in patients treated with heparin is important for the early detection of HIT. Treatment consists of discontinuation of heparin and alternative anticoagulant treatment, if still required.
Chronic thromboembolic pulmonary hypertension
CTEPH is a relatively rare complication of PE.367 In patients with CTEPH, the original embolic material is replaced over a period of months to years with fibrous tissue that is incorporated into the intima and media of the pulmonary arteries. This material may extend to the segmental and subsegmental branches of the pulmonary arteries. Partial recanalization or total occlusion of the involved pulmonary artery vasculature may occur.
The chronic obstruction of the pulmonary vascular bed is followed by progressive elevation of pulmonary artery resistance, ultimately leading to right heart failure.274 The initial phase of the disease is often asymptomatic, but is followed by progressive dyspnoea and hypoxaemia. In the late phase of the disease, patients may have all the signs of advanced right heart failure. CTEPH should be suspected in every patient with pulmonary hypertension.368 The diagnostic strategy is based on echocardiography, perfusion scintigraphy, CT, right heart catheterization and pulmonary angiography.369 Medical therapy aims to treat right heart failure and to lower pulmonary artery resistance. Preliminary data suggest some haemodynamic and/or functional improvement with prostacyclin analogues, endothelin receptor antagonists and phosphodiesterase-5 inhibitors. However, the efficacy of any medical therapy is limited by the morphological substrate of pulmonary artery obstruction. Therefore, potential future candidates for chronic medical treatment in CTEPH include non-operable patients and patients in whom surgical intervention has failed to restore near-normal haemodynamics.
Pulmonary thromboendarterectomy (endarterectomy) was first introduced in 1957 and has since then evolved to become a relatively common treatment for CTEPH. Selection criteria for pulmonary thromboendarterectomy have been defined by the guidelines of the American College of Chest Physicians370 and include: (i) New York Heart Association (NYHA) functional class III or IV symptoms; (ii) preoperative pulmonary vascular resistance greater than 300 dyn s cm−5; (iii) surgically accessible thrombi in the main, lobar or segmental pulmonary arteries; and (iv) absence of severe comorbidity.
Surgical removal of the obstructing material requires a true endarterectomy as opposed to a simple embolectomy.371 For this reason the operation is performed on cardiopulmonary bypass, with deep hypothermia and complete circulatory arrest in order to provide adequate visibility. The main pulmonary arteries are incised and the right endarterectomy level within the wall is defined. Thereafter, the plane is followed circumferentially down to the segmental and sometimes subsegmental branches of each lobar artery, a procedure which is performed with the help of special suction dissectors.372
As there is currently no preoperative classification system for CTEPH, patients with CTEPH can be postoperatively classified into four categories according to the location and type of the lesions found during operation.373 Type 1 is characterized by a fresh thrombus in the main lobar pulmonary arteries; type 2 by thickening and fibrosis of the intima proximally to the segmental arteries; type 3 by the involvement of distal segmental arteries only; and type 4 by distal arteriolar involvement without visible thromboembolic disease.
Perioperative mortality is related to the severity of the disease, with a mortality rate of 4% in patients with a preoperative pulmonary vascular resistance less than 900 dyn s cm−5 and 20% in those with pulmonary vascular resistance above 1200 dyn s cm−5. The functional results of a successful pulmonary thromboendarterectomy are excellent and generally sustained over time,374,375 with a 3-year survival rate of about 80%.376 Although recent data have demonstrated a 2-year cumulative incidence of 3.8% for CTEPH after a symptomatic PE,377 no recommendations can be made yet regarding screening for CTEPH in PE survivors.
In summary, CTEPH is a severe though rare consequence of PE. Pulmonary endarterectomy provides excellent results and should be considered as a first-line treatment whenever possible. Drugs targeting the pulmonary circulation in patients in whom surgery is not feasible or has failed are currently being tested in clinical trials.
Non-thrombotic pulmonary embolism
Septic embolism
Septic embolism to the pulmonary circulation is a relatively rare clinical event. Septic pulmonary emboli are most commonly associated with tricuspid valve endocarditis, mainly occurring in drug addicts378 but also in patients with infected indwelling catheters and pacemaker wires,379 and in patients with peripheral septic thrombophlebitis or organ transplants.380 Typically, patients present with fever, cough and haemoptysis. Antibiotic treatment is generally successful; however, occasionally the source of emboli must be removed surgically.381
Intravascular foreign bodies
Several types of intravascular foreign bodies can embolize to the pulmonary arteries. They include broken catheters, guidewires and vena cava filters382–384 and, more recently, coils for embolization and endovascular stent components. Most intravascular foreign bodies are found in the pulmonary arteries, and the remainder in the right heart or the vena cava.385 Intravascular retrieval using snares is frequently successful.386,387
Fat embolism
The fat embolism syndrome is a combination of respiratory, haematological, neurological and cutaneous symptoms and signs associated with trauma and several other surgical and medical conditions. The incidence of the clinical syndrome is low (<1%), while the embolization of marrow fat appears to be an almost inevitable consequence of long bone fractures.388 The presentation may be fulminating with pulmonary and systemic embolization of fat, right ventricular failure and cardiovascular collapse.389 More usually, the onset is gradual, with hypoxaemia, neurological symptoms, fever and a petechial rash, typically 12–36 h after injury.390 Fat embolism is reported in many other conditions,388 such as liposuction,391 lipid and propofol infusions,392 and in patients with hepatic necrosis and fatty liver.393
The pathogenesis of fat embolism syndrome is not completely understood.394 Treatment is non-specific and supportive.388
Venous air embolism
Vascular air embolism is the entrainment of air (or exogenously delivered gas) from the operative field or other communication with the environment into the venous or arterial vasculature, producing systemic effects.395 The morbidity and mortality rates of vascular air embolism are directly related to the volume of air entrainment and rate of accumulation. From case reports of accidental intravascular delivery of air, the adult lethal volume has been described as between 200 and 300 ml, or 3–5 ml/kg396 injected at a rate of 100 ml/s.397
The major effect of venous air embolism is the obstruction of the right ventricular pulmonary outflow tract or obstruction of the pulmonary arterioles by a mixture of air bubbles and fibrin clots formed in the heart. The result in either situation is cardiovascular dysfunction and failure. Principal goals of management include prevention of further air entry, a reduction in the volume of air entrained, if possible, and haemodynamic support.395
Patients with suspected venous air embolism should be placed in the left lateral decubitus head-down position. Occasionally, intraoperative needle aspiration is performed to relieve large air bubbles.394,395
There have been numerous case reports and case series illustrating the potential benefits of hyperbaric oxygen therapy, especially in the presence of cerebral arterial gas embolism.395
Amniotic fluid embolism
Amniotic fluid embolism is a rare but catastrophic complication unique to pregnancy. Amniotic emboli occur in 1/8000–1/80 000 pregnancies; however, the emboli result in high maternal and fetal mortality rates (80 and 40%, respectively). It is a complex phenomenon, ranging from mild degree of organ dysfunction to coagulopathy, cardiovascular collapse and death.
This condition occurs when amniotic fluid is forced into the bloodstream through small tears in the uterine veins during normal labour. Dyspnoea, cyanosis and shock that are abrupt in onset classically progress rapidly to cardiopulmonary collapse and severe pulmonary oedema. The pathophysiology of amniotic fluid embolism is multifactorial and poorly understood. The diagnosis is one of exclusion and management is supportive.398
Talc embolism
Many substances, such as magnesium trisilicate (talc), starch and cellulose, are used as fillers in drug manufacturing. Some of these drugs (prepared as oral medications), such as amphetamines, methylphenidate, hydromorphone and dextropropoxyphene, are ground by drug users, mixed in liquid, and injected intravenously. These filler particles are mainly entrapped within the pulmonary vasculature and can cause thrombosis and the formation of intravascular granulomata.
Tumour embolism
Pulmonary intravascular tumour emboli are seen in up to 26% of autopsies but are much less frequently identified before death.399 Pulmonary tumour embolism radiologically mimics pneumonia, tuberculosis or interstitial lung disease. Intracardiac source of pulmonary tumour emboli may be diagnosed by imaging methods. In a review of microscopic pulmonary tumour emboli associated with dyspnoea, Kane et al. found that carcinomas of the prostate gland and breast were the most common causes, followed by hepatoma, then carcinomas of the stomach and pancreas.400 Treatment for this entity has not been studied extensively, since the diagnosis is usually not made until the post mortem. However there are reports of limited success with chemotherapy.
Rare causes
There are several reports describing rare causes of non-thrombotic PE: cotton embolism, hydatid embolism, iodinated oil embolism, metallic mercury embolism and cement (polymethylmethacrylate) embolism may account for more or less severe PE with great variability of symptoms.
In summary, non-thrombotic PE does not represent a distinct clinical syndrome. It may be due to a variety of embolic materials and result in a wide spectrum of clinical presentations, making the diagnosis difficult. With the exception of severe air and fat embolism, the haemodynamic consequences of non-thrombotic emboli are usually mild. Treatment is mostly supportive but may differ according to the type of embolic material and clinical severity.
Supplementary material
The CME text ‘Guidelines on the diagnosis and management of acute pulmonary embolism’ is accredited by the European Board for Accreditation in Cardiology (EBAC) for ‘2’ hours of External CME credits. Each participant should claim only those hours of credit that have actually been spent in the educational activity. EBAC works in cooperation with the European Accreditation Council for Continuing Medical Education (EACCME), which is an institution of the European Union of Medical Specialists (UEMS). In compliance with EBAC/EACCME guidelines, all authors participating in this programme have disclosed potential conflicts of interest that might cause a bias in the article. The Organizing Committee is responsible for ensuring that all potential conflicts of interest relevant to the programme are declared to the participants prior to the CME activities. CME questions for this article are available at the web sites of the European Heart Journal (http://cme.oxfordjournals.org/cgi/hierarchy/oupcme_node;ehj) and the European Society of Cardiology (http://www.escardio.org/knowledge/guidelines).
List of acronyms and abbreviations
- aPTT
activated partial thromboplastin time
- anti-Xa
anti-factor Xa activity
- BNP
brain natriuretic peptide
- CI
confidence interval
- CT
computed tomography
- CTEPH
chronic thromboembolic pulmonary hypertension
- CUS
compression venous ultrasonography
- DVT
deep vein thrombosis
- ECG
electrocardiogram
- ELISA
enzyme-linked immunoabsorbent assay
- HIT
heparin-induced thrombocytopenia
- ICOPER
International Cooperative Pulmonary Embolism Registry
- INR
international normalized ratio
- IVC
inferior vena cava
- LMWH
low molecular weight heparin
- LV
left ventricle
- MDCT
multidetector computed tomography
- NPV
negative predictive value
- NT-proBNP
N-terminal proBNP
- OR
odds ratio
- PaO2
arterial oxygen pressure
- PE
pulmonary embolism
- PIOPED
Prospective Investigation On Pulmonary Embolism Diagnosis study
- PPV
positive predictive value
- rtPA
recombinant tissue plasminogen activator
- RV
right ventricle
- RVD
right ventricular dysfunction
- SBP
systolic blood pressure
- SDCT
single-detector computed tomography
- VKA
vitamin K antagonist
- VTE
venous thromboembolism
- V/Q scan
ventilation–perfusion scintigraphy
References
Author notes
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC.
Disclaimer. The ESC Guidelines represent the views of the ESC and were arrived at after careful consideration of the available evidence at the time they were written. Health professionals are encouraged to take them fully into account when exercising their clinical judgement. The guidelines do not, however, override the individual responsibility of health professionals to make appropriate decisions in the circumstances of the individual patients, in consultation with that patient, and where appropriate and necessary the patient's guardian or carer. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.



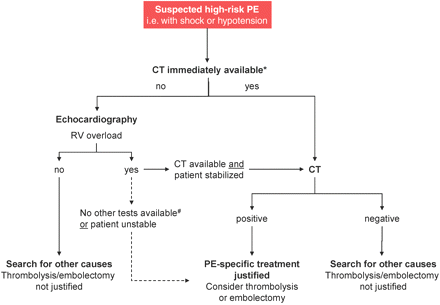
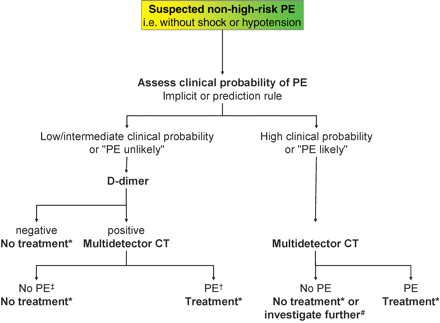

Comments
We would like to thank Dr Nachman for his interesting remarks. It is rewarding to see that our document is still alive, read and commented after 3 years since its publication. While ventilation -perfusion (V/Q) lung scan was not recommended by the guidelines as a first choice diagnostic strategy due to the overwhelming success of CT angiography in recent years, it still remains useful in many hospitals throughout Europe. The interpretation of normal or near-normal V/Q scans which adequately rule out pulmonary embolism, (PE) and of high probability scans which establish the diagnosis, is straightforward. On the other hand, non- diagnostic scan results (i.e. low or intermediate probability according to the PIOPED classification), remain a challenge and cannot rule out PE without taking clinical probability into account. The prevalence of PE in patients with a low clinical probability and a low probability V/Q scan was only 4% in the classical PIOPED study (1) and only 2% in an analysis of several series from the Geneva group.(2) Hence, the value of ruling out PE in a patients with a low clinical probability and a low probability V/Q scan is well established and has been endorsed by several guidelines. However, when low- and intermediate-probability V/Q scans are grouped together into a "non-diagnostic" category, the prevalence of PE in patients with a low clinical probability and a non-diagnostic scan is higher; in a pooled analysis of the Geneva and the PIOPED series, it was approximately 6% (95%CI 4 to 9%).(1-2) This proportion of "false-negative" tests may be too high to firmly recommend this combination as a valid criterion for ruling out PE. Therefore, most recent algorithms propose performing a proximal lower limb ultrasound in patients with a non- diagnostic V/Q scan and a low clinical probability. If deep venous thrombosis is absent, several well conducted management studies have shown that anticoagulant can be safely whithheld.(3-4) In patients with an intermediate clinical probability and the same combination (non-diagnostic V/Q scan and negative ultrasound), the post-test probability of PE is also low but the evidence from management trials is more scarce. In summary, we recognize that our recommendation may be somewhat ambiguous and should be read as follows: In patients with a low clinical probability, the post-test probability of PE after a non-diagnostic V/Q is low. This combination may be used to rule out PE. However, the addition of a negative proximal lower limb ultrasonography further reduces the post- test probability of PE and is a safer criterion to rule out PE. In the table mentioned by Dr Nachman, our message was that in patients with a low clinical probability, a non-conclusive scan may justify withholding treatment "particularly in case of a negative CUS examination", with the level of recommendation increasing from IIa-B to I-A, respectively. Finally, in patients with an intermediate clinical probability and a non- diagnostic V/Q scan, PE may only be ruled out in combination with a negative lower limb ultrasonography.
References
1. The PIOPED Investigators. Value of the ventilation-perfusion scan in acute pulmonary embolism. JAMA. 1990;263:2753-9.
2. Perrier A, Miron MJ, Desmarais S, de Moerloose P, Slosman D, Didier D, et al. Using clinical evaluation and lung scan to rule out suspected pulmonary embolism: Is it a valid option in patients with normal results of lower- limb venous compression ultrasonography? Arch Intern Med. 2000;160(4):512-6.
3. Anderson DR, Kahn SR, Rodger MA, Kovacs MJ, Morris T, Hirsch A, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007 Dec 19;298(23):2743-53.
4. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135(2):98-107.
Conflict of Interest:
None declared
I congratulate the Task force member on their valuable guideline. In your report, Table 10 suggested that non diagnostic perfusion scan, as the sole diagnostic test, is valid to exclude Pulmonary Embolism (PE) in people with low clinical probability.
In the Prospective Investigation for Pulmonary Embolism Dagnosis (1), the Probability of PE in this group was 19%. Thus, PE cannot be safely ruled out with a non diagnostic perfusion scan as the sole test. Stein et al.(2) proposed a strategy of diagnosis based on lung scans and noninvasive lower extremity imaging. With non diagnostic lung san and low clinical probability a negative lower extremity study would decrease the chance of having PE to 9%, a probability that may allow withholding anticoagulation safely. This is the approach that was clinically validated with an outcome study by Wells et al.(3)
Certainly a normal or near normal perfusion scan combined with low clinical probability can safely exclude PE, however, this is not the case with non diagnostic perfusion scan.
References
1. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA 1990;263:2753-9.
2. Stein PD, Hull RD, Saltzman HA, Pineo G. Strategy for diagnosis of patients with suspected acute pulmonary embolism. Chest 1993;103:1553-9.
3. Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 1998;129:997-1005.
Conflict of Interest:
None declared