-
PDF
- Split View
-
Views
-
Cite
Cite
Eric Bonnefoy, Philippe Gabriel Steg, Florent Boutitie, Pierre-Yves Dubien, Frédéric Lapostolle, Jérome Roncalli, Frederic Dissait, Gérald Vanzetto, Alain Leizorowicz, Gilbert Kirkorian, for the CAPTIM Investigators, Comparison of primary angioplasty and pre-hospital fibrinolysis in acute myocardial infarction (CAPTIM) trial: a 5-year follow-up, European Heart Journal, Volume 30, Issue 13, July 2009, Pages 1598–1606, https://doi.org/10.1093/eurheartj/ehp156
Close - Share Icon Share
Abstract
The CAPTIM (Comparison of primary Angioplasty and Pre-hospital fibrinolysis In acute Myocardial infarction) study found no evidence that a strategy of primary angioplasty was superior in terms of 30-day outcomes to a strategy of pre-hospital fibrinolysis with transfer to an interventional facility in patients managed early at the acute phase of an acute myocardial infarction. The present analysis was designed to compare both strategies at 5 years.
The CAPTIM study included 840 patients managed in a pre-hospital setting within 6 h of an acute ST-segment elevation myocardial infarction. Patients were randomized to either a primary angioplasty (n = 421) or a pre-hospital fibrinolysis (rt-PA) with immediate transfer to a centre with interventional facilities (n = 419). Long-term follow-up was obtained in blinded fashion from 795 patients (94.6%). Using an intent-to-treat analysis, all-cause mortality at 5 years was 9.7% in the pre-hospital fibrinolysis group when compared with 12.6% in the primary angioplasty group [HR 0.75 (95% CI, 0.50–1.14); P = 0.18]. For patients included within 2 h, 5 year mortality was 5.8% in the pre-hospital fibrinolysis group when compared with 11.1% in the primary angioplasty group [HR 0.50 (95% CI, 0.25–0.97); P = 0.04], whereas it was, respectively, 14.5 and 14.4% in patients included after 2 h [HR 1.02, (95% CI 0.59–1.75), P = 0.92].
The 5-year follow-up is consistent with the 30-day outcomes of the trial, showing similar mortality for primary percutaneous coronary intervention and a policy of pre-hospital lysis followed by transfer to an interventional center. In addition, for patients treated within 2 h of symptom onset, 5-year mortality was lower with pre-hospital lysis.
Introduction
Reperfusion strategies at the early phase of acute myocardial infarction with ST-segment elevation with primary angioplasty or fibrinolysis had been addressed in numerous studies and in different settings.
For patients presenting directly to a hospital with on-site interventional cardiology, mechanical reperfusion by primary angioplasty has been shown to reduce the short-term risk of death, recurrent infarction, and stroke, when compared with in-hospital fibrinolysis.1 Similar benefits have been observed in patients initially managed in a general hospital and transferred to an interventional cardiology centre for primary angioplasty.2,3 These results are consistent between studies and are maintained at long-term follow-up.4,5
However, for the population of patients managed in the pre-hospital setting, the benefit of primary angioplasty over pre-hospital fibrinolysis is not as clear cut. The CAPTIM study was designed to compare pre-hospital fibrinolysis, with transfer to a centre with interventional cardiology for rescue angioplasty if needed, and primary angioplasty in patients with an acute ST-segment elevation myocardial infarction. In this study, a trend was observed towards a lower mortality at 30 days in the pre-hospital fibrinolysis group,6 especially in patients managed within the first 2 h.7
The aim of the present analysis was to investigate the long-term (5-year) mortality of the patients included in the CAPTIM study according to the initial strategy.
Methods
Study patients
The CAPTIM study has been described in detail previously (CAPTIM). Briefly, out-of-hospital patients were managed by mobile intensive care units (MICUs) that included a physician and carried an ECG and resuscitation equipment. The patients were eligible for inclusion if they presented within 6 h after the onset of symptoms of myocardial infarction, i.e. characteristic pain lasting for at least 30 min, or pain lasting for <30 min but still present and not responsive to nitrates, and with electrocardiographic ST-segment elevation of at least 0.2 mV in two or more contiguous leads or left bundle-branch block. Patients were excluded if they were known to have haemorrhagic diathesis or any contraindication to fibrinolysis, severe renal or hepatic insufficiency, aorto-femoral bypass or any condition that could hamper femoral artery access, cardiogenic shock, history of coronary artery bypass (CABG), or were receiving oral anticoagulant treatment. They could also be excluded if the duration of transfer to the hospital was expected to exceed 1 h.
Randomization and treatment strategies
Eligible patients were randomly assigned at the site of initial management (usually at home or at their workplace) to the pre-hospital rt-PA or primary angioplasty treatment group. The MICU teams were in permanent radio contact with the MICU medical dispatcher who performed randomization using a central, 24 h, computerized, randomization service. Patients assigned to pre-hospital fibrinolysis received a 5000 U intravenous (IV) heparin bolus, 250–500 mg aspirin (orally or IV), and an IV bolus of 15 mg alteplase (rt-PA). This was followed by an rt-PA infusion of 0.75 mg/kg of body weight (not to exceed 50 mg) over a 30 min period and then 0.50 mg/kg (not to exceed 35 mg) over the next 60 min, up to a maximal total dose of 100 mg. Treatment was started by the emergency physician at the site of the intervention. All patients were then transported to the hospital.
The decision to perform angiography in the hospital was left to the judgment of the investigator.
Primary angioplasty patients received a 5000 U IV bolus of heparin and 250–500 mg of aspirin (orally or IV) and were transported immediately to the hospital for coronary angiography and, if indicated, angioplasty. Angioplasty was performed according to local standards with the intention of re-establishing blood flow in the infarct-related artery as soon as possible. The infarct-related artery was the only target, except in patients whose haemodynamic parameters deteriorated despite restoration of the patency of that artery. The study protocol advised that in patients with stenoses of the left main stem or critical three-vessel disease, CABG should be strongly considered in place of angioplasty.
Endpoints
The primary endpoint of the original trial was a composite of death, non-fatal re-infarction, and non-fatal disabling stroke within 30 days. The primary endpoint of this analysis was mortality at 5 years of follow up.
The 5-year follow up was established through hospital records, phone contact with relatives of the patient or attending physician, and registries of the patient’s birthplace. It was complete in all but 45 patients [5.4% of the entire population; 17 patients in the pre-hospital strategy group and 28 patients in the percutaneous coronary intervention (PCI) group]. The median length (1st–3rd quartile) of the follow up interval for those patients who could not be contacted at 5 years was 2.0 years [1.3–2.6], with a range between 0.1 and 4.8 years.
Statistical analysis
Two subgroup analyses, on delay to presentation and diabetes, were considered of special interest to the Steering Committee and were selected on an a priori basis. These analyses have been previously published6,7,12 for the 1-month outcome. Therefore, the 5-year survival analysis of the CAPTIM study was extended to these two subgroups. Continuous data are presented as medians with 1st and 3rd quartiles unless otherwise stated. The Kaplan–Meier method was used to estimate and plot survival probabilities in the two treatment groups at 1 month, 1, 3, and 5 years. Fatal events occurring after 5 years of follow-up were censored. The proportional hazard model was used to estimate the hazard ratio and 95% confidence interval of mortality in the pre-hospital fibrinolysis group over that in the PCI group. Hazard ratios were estimated at 5 years of follow-up using independent models. Interaction between randomized treatment assignment and diabetes was examined in a Cox model in which these two variables and their interaction were entered. The same has been done for time to treatment. Tests of significance were two-tailed. P-value of <0.05 was considered statistically significant, and statistical analysis was performed on the basis of intention-to-treat. SAS software (Windows V 9.1) was used for all analyses.
Results
Basic characteristics of the patients included in the CAPTIM trial are shown in Table 1. The 30-day clinical results of the initial CAPTIM trial have previously been reported. There was no difference in the combined end point of death, re-infarction, and disabling strokes.6
Baseline characteristics of patients
| . | Pre-hospital fibrinolysis (n = 419) . | Primary angioplasty (n = 421) . | P-value . |
|---|---|---|---|
| Characteristic | |||
| Age, years | 58 (49–69) | 58 (50–68) | 0.69 |
| Age >75 years | 42/419 (10.0%) | 40/421 (9.5%) | 0.80 |
| Male sex | 345/419 (82.3%) | 343/421 (81.5%) | 0.48 |
| Current smoker | 216/411 (52.6%) | 205/417 (49.2%) | 0.48 |
| Diabetes | 46/416 (11.1%) | 57/418 (13.6%) | 0.29 |
| Hypertension | 141/416 (33.9%) | 146/419 (34.8%) | 0.83 |
| Dyslipidaemia | 212/415 (51.1%) | 215/418 (51.4%) | 0.94 |
| Prior bypass surgery | 0/416 (0.0%) | 5/416 (1.2%) | 0.06 |
| Prior myocardial infarction | 34/416 (8.2%) | 28/418 (6.7%) | 0.43 |
| Prior angioplasty | 22/416 (5.3%) | 18/418 (4.3%) | 0.52 |
| Heart rate, b.p.m. | 75 (64–84) | 75 (66–88) | 0.13 |
| Systolic blood pressure, mmHg | 125 (110–140) | 128 (111–140) | 0.56 |
| Anterior infarction | 166/413 (40.2%) | 178/417 (42.7%) | 0.48 |
| Time to randomization, min | 107 (76–158) | 108 (76–162) | 0.96 |
| Time to treatment, min | 130 (95–180) | 190 (149–255) | <0.001 |
| Medication or procedure | |||
| Angiotensin-converting enzyme inhibitor | 212/394 (53.8%) | 194/394 (49.2%) | 0.23 |
| Aspirin | 388/405 (95.8%) | 395/406 (97.3%) | 0.26 |
| Beta-blocker | 358/385 (93.0%) | 334/387 (86.3%) | 0.003 |
| Calcium-channel blocker | 35/405 (8.6%) | 41/406 (10.1%) | 0.55 |
| Statin | 231/405 (57.0%) | 222/406 (54.7%) | 0.52 |
| Ticlopidine/clopidogrel | 241/405 (59.5%) | 307/406 (75.6%) | <0.001 |
| Urgent angioplasty for persistent ischaemia (rescue) | 106/407a (26.0%) | 7/412 (1.7%) | <0.001 |
| Urgent angioplasty for recurrent ischaemia | 28/418 (6.7%) | 9/420 (2.1%) | 0.001 |
| Coronary-artery bypass surgery | 6/401 (1.5%) | 3/401 (0.7%) | 0.51 |
| . | Pre-hospital fibrinolysis (n = 419) . | Primary angioplasty (n = 421) . | P-value . |
|---|---|---|---|
| Characteristic | |||
| Age, years | 58 (49–69) | 58 (50–68) | 0.69 |
| Age >75 years | 42/419 (10.0%) | 40/421 (9.5%) | 0.80 |
| Male sex | 345/419 (82.3%) | 343/421 (81.5%) | 0.48 |
| Current smoker | 216/411 (52.6%) | 205/417 (49.2%) | 0.48 |
| Diabetes | 46/416 (11.1%) | 57/418 (13.6%) | 0.29 |
| Hypertension | 141/416 (33.9%) | 146/419 (34.8%) | 0.83 |
| Dyslipidaemia | 212/415 (51.1%) | 215/418 (51.4%) | 0.94 |
| Prior bypass surgery | 0/416 (0.0%) | 5/416 (1.2%) | 0.06 |
| Prior myocardial infarction | 34/416 (8.2%) | 28/418 (6.7%) | 0.43 |
| Prior angioplasty | 22/416 (5.3%) | 18/418 (4.3%) | 0.52 |
| Heart rate, b.p.m. | 75 (64–84) | 75 (66–88) | 0.13 |
| Systolic blood pressure, mmHg | 125 (110–140) | 128 (111–140) | 0.56 |
| Anterior infarction | 166/413 (40.2%) | 178/417 (42.7%) | 0.48 |
| Time to randomization, min | 107 (76–158) | 108 (76–162) | 0.96 |
| Time to treatment, min | 130 (95–180) | 190 (149–255) | <0.001 |
| Medication or procedure | |||
| Angiotensin-converting enzyme inhibitor | 212/394 (53.8%) | 194/394 (49.2%) | 0.23 |
| Aspirin | 388/405 (95.8%) | 395/406 (97.3%) | 0.26 |
| Beta-blocker | 358/385 (93.0%) | 334/387 (86.3%) | 0.003 |
| Calcium-channel blocker | 35/405 (8.6%) | 41/406 (10.1%) | 0.55 |
| Statin | 231/405 (57.0%) | 222/406 (54.7%) | 0.52 |
| Ticlopidine/clopidogrel | 241/405 (59.5%) | 307/406 (75.6%) | <0.001 |
| Urgent angioplasty for persistent ischaemia (rescue) | 106/407a (26.0%) | 7/412 (1.7%) | <0.001 |
| Urgent angioplasty for recurrent ischaemia | 28/418 (6.7%) | 9/420 (2.1%) | 0.001 |
| Coronary-artery bypass surgery | 6/401 (1.5%) | 3/401 (0.7%) | 0.51 |
Continuous data are presented as medians with 1st and 3rd quartiles. The varying denominators for categorical data reflect missing data.
aIncludes the 19 patients who had no thrombolysis (5 of them had primary angioplasty).
Baseline characteristics of patients
| . | Pre-hospital fibrinolysis (n = 419) . | Primary angioplasty (n = 421) . | P-value . |
|---|---|---|---|
| Characteristic | |||
| Age, years | 58 (49–69) | 58 (50–68) | 0.69 |
| Age >75 years | 42/419 (10.0%) | 40/421 (9.5%) | 0.80 |
| Male sex | 345/419 (82.3%) | 343/421 (81.5%) | 0.48 |
| Current smoker | 216/411 (52.6%) | 205/417 (49.2%) | 0.48 |
| Diabetes | 46/416 (11.1%) | 57/418 (13.6%) | 0.29 |
| Hypertension | 141/416 (33.9%) | 146/419 (34.8%) | 0.83 |
| Dyslipidaemia | 212/415 (51.1%) | 215/418 (51.4%) | 0.94 |
| Prior bypass surgery | 0/416 (0.0%) | 5/416 (1.2%) | 0.06 |
| Prior myocardial infarction | 34/416 (8.2%) | 28/418 (6.7%) | 0.43 |
| Prior angioplasty | 22/416 (5.3%) | 18/418 (4.3%) | 0.52 |
| Heart rate, b.p.m. | 75 (64–84) | 75 (66–88) | 0.13 |
| Systolic blood pressure, mmHg | 125 (110–140) | 128 (111–140) | 0.56 |
| Anterior infarction | 166/413 (40.2%) | 178/417 (42.7%) | 0.48 |
| Time to randomization, min | 107 (76–158) | 108 (76–162) | 0.96 |
| Time to treatment, min | 130 (95–180) | 190 (149–255) | <0.001 |
| Medication or procedure | |||
| Angiotensin-converting enzyme inhibitor | 212/394 (53.8%) | 194/394 (49.2%) | 0.23 |
| Aspirin | 388/405 (95.8%) | 395/406 (97.3%) | 0.26 |
| Beta-blocker | 358/385 (93.0%) | 334/387 (86.3%) | 0.003 |
| Calcium-channel blocker | 35/405 (8.6%) | 41/406 (10.1%) | 0.55 |
| Statin | 231/405 (57.0%) | 222/406 (54.7%) | 0.52 |
| Ticlopidine/clopidogrel | 241/405 (59.5%) | 307/406 (75.6%) | <0.001 |
| Urgent angioplasty for persistent ischaemia (rescue) | 106/407a (26.0%) | 7/412 (1.7%) | <0.001 |
| Urgent angioplasty for recurrent ischaemia | 28/418 (6.7%) | 9/420 (2.1%) | 0.001 |
| Coronary-artery bypass surgery | 6/401 (1.5%) | 3/401 (0.7%) | 0.51 |
| . | Pre-hospital fibrinolysis (n = 419) . | Primary angioplasty (n = 421) . | P-value . |
|---|---|---|---|
| Characteristic | |||
| Age, years | 58 (49–69) | 58 (50–68) | 0.69 |
| Age >75 years | 42/419 (10.0%) | 40/421 (9.5%) | 0.80 |
| Male sex | 345/419 (82.3%) | 343/421 (81.5%) | 0.48 |
| Current smoker | 216/411 (52.6%) | 205/417 (49.2%) | 0.48 |
| Diabetes | 46/416 (11.1%) | 57/418 (13.6%) | 0.29 |
| Hypertension | 141/416 (33.9%) | 146/419 (34.8%) | 0.83 |
| Dyslipidaemia | 212/415 (51.1%) | 215/418 (51.4%) | 0.94 |
| Prior bypass surgery | 0/416 (0.0%) | 5/416 (1.2%) | 0.06 |
| Prior myocardial infarction | 34/416 (8.2%) | 28/418 (6.7%) | 0.43 |
| Prior angioplasty | 22/416 (5.3%) | 18/418 (4.3%) | 0.52 |
| Heart rate, b.p.m. | 75 (64–84) | 75 (66–88) | 0.13 |
| Systolic blood pressure, mmHg | 125 (110–140) | 128 (111–140) | 0.56 |
| Anterior infarction | 166/413 (40.2%) | 178/417 (42.7%) | 0.48 |
| Time to randomization, min | 107 (76–158) | 108 (76–162) | 0.96 |
| Time to treatment, min | 130 (95–180) | 190 (149–255) | <0.001 |
| Medication or procedure | |||
| Angiotensin-converting enzyme inhibitor | 212/394 (53.8%) | 194/394 (49.2%) | 0.23 |
| Aspirin | 388/405 (95.8%) | 395/406 (97.3%) | 0.26 |
| Beta-blocker | 358/385 (93.0%) | 334/387 (86.3%) | 0.003 |
| Calcium-channel blocker | 35/405 (8.6%) | 41/406 (10.1%) | 0.55 |
| Statin | 231/405 (57.0%) | 222/406 (54.7%) | 0.52 |
| Ticlopidine/clopidogrel | 241/405 (59.5%) | 307/406 (75.6%) | <0.001 |
| Urgent angioplasty for persistent ischaemia (rescue) | 106/407a (26.0%) | 7/412 (1.7%) | <0.001 |
| Urgent angioplasty for recurrent ischaemia | 28/418 (6.7%) | 9/420 (2.1%) | 0.001 |
| Coronary-artery bypass surgery | 6/401 (1.5%) | 3/401 (0.7%) | 0.51 |
Continuous data are presented as medians with 1st and 3rd quartiles. The varying denominators for categorical data reflect missing data.
aIncludes the 19 patients who had no thrombolysis (5 of them had primary angioplasty).
Figure 1 shows the survival curves for the two groups over the 5-year follow-up period. The overall mortality was 10.9% (92 patients). On the basis of the Kaplan–Meier analysis, overall mortality was 9.7% (40 patients) in the pre-hospital fibrinolysis strategy group and 12.6% (52 patients) in the primary PCI group [hazard ratio, 0.75 (95% CI, 0.50–1.14); P = 0.18].
Survival according to randomized treatment assignment. [Pre-hospital fibrinolysis group (broken line) and PCI group (solid line).] Underneath the graph are the numbers of patients at risk for each time point (those who survived and had been at least followed up to this point).
For patients who died, the median interval to death was 6.7 months (1st–3rd quartile, 0.08–31.42) in the primary PCI group and 3.8 months (1st–3rd quartile, 0.11–33.79) in the fibrinolysis group.
The 1-month and 1-, 3-, and 5-year death rates connected with the treatment strategy are presented in Table 2 and Figure 2 for the entire population, for patients managed within 2 h or after 2 h from symptom onset and for diabetics and non diabetics.
Survival according to randomized treatment assignment in diabetics and non-diabetics. [Pre-hospital fibrinolysis group (broken line) and PCI group (solid line).] Underneath the graph are the numbers of patients at risk for each time point.
Impact of treatment strategy on mortality in the global population, in patients managed within and after 2 h, in diabetics and non-diabetics according to time of follow-up
| . | Cumulative number of deaths fibrinolysis /angioplasty . | Pre-hospital fibrinolysis (%) . | Primary angioplasty (%) . | Hazard ratio (95% confidence interval) . | P-value . | *P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| 1 month | 16/20 | 3.8 | 4.8 | |||
| 1 year | 23/30 | 5.5 | 7.1 | |||
| 3 years | 31/40 | 7.5 | 9.6 | |||
| 5 years | 40/52 | 9.7 | 12.6 | 0.75 (0.50–1.14) | 0.18 | |
| <2 h | ||||||
| 1 month | 5/13 | 2.2 | 5.7 | |||
| 1 year | 7/17 | 3.0 | 7.4 | |||
| 3 years | 8/21 | 3.5 | 9.2 | |||
| 5 years | 13/25 | 5.8 | 11.1 | 0.50 (0.25–0.98) | 0.04 | |
| >2 h | ||||||
| 1 month | 11/7 | 5.9 | 3.7 | |||
| 1 year | 16/13 | 8.6 | 7.0 | |||
| 3 years | 23/19 | 12.3 | 10.3 | |||
| 5 years | 27/26 | 14.5 | 14.4 | 1.02 (0.59–1.75) | 0.92 | 0.10* |
| Diabetics | ||||||
| 1 month | 6/3 | 13.0 | 5.3 | |||
| 1 year | 8/6 | 17.6 | 10.5 | |||
| 3 years | 8/10 | 17.6 | 17.9 | |||
| 5 years | 9/12 | 20.0 | 21.7 | 0.99 (0.42–2.36) | 0.99 | |
| Non-diabetics | ||||||
| 1 month | 9/14 | 2.4 | 3.9 | |||
| 1 year | 14/21 | 3.8 | 5.8 | |||
| 3 years | 22/27 | 6.0 | 7.5 | |||
| 5 years | 30/37 | 8.2 | 10.5 | 0.77 (0.42–1.25) | 0.29 | 0.64* |
| . | Cumulative number of deaths fibrinolysis /angioplasty . | Pre-hospital fibrinolysis (%) . | Primary angioplasty (%) . | Hazard ratio (95% confidence interval) . | P-value . | *P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| 1 month | 16/20 | 3.8 | 4.8 | |||
| 1 year | 23/30 | 5.5 | 7.1 | |||
| 3 years | 31/40 | 7.5 | 9.6 | |||
| 5 years | 40/52 | 9.7 | 12.6 | 0.75 (0.50–1.14) | 0.18 | |
| <2 h | ||||||
| 1 month | 5/13 | 2.2 | 5.7 | |||
| 1 year | 7/17 | 3.0 | 7.4 | |||
| 3 years | 8/21 | 3.5 | 9.2 | |||
| 5 years | 13/25 | 5.8 | 11.1 | 0.50 (0.25–0.98) | 0.04 | |
| >2 h | ||||||
| 1 month | 11/7 | 5.9 | 3.7 | |||
| 1 year | 16/13 | 8.6 | 7.0 | |||
| 3 years | 23/19 | 12.3 | 10.3 | |||
| 5 years | 27/26 | 14.5 | 14.4 | 1.02 (0.59–1.75) | 0.92 | 0.10* |
| Diabetics | ||||||
| 1 month | 6/3 | 13.0 | 5.3 | |||
| 1 year | 8/6 | 17.6 | 10.5 | |||
| 3 years | 8/10 | 17.6 | 17.9 | |||
| 5 years | 9/12 | 20.0 | 21.7 | 0.99 (0.42–2.36) | 0.99 | |
| Non-diabetics | ||||||
| 1 month | 9/14 | 2.4 | 3.9 | |||
| 1 year | 14/21 | 3.8 | 5.8 | |||
| 3 years | 22/27 | 6.0 | 7.5 | |||
| 5 years | 30/37 | 8.2 | 10.5 | 0.77 (0.42–1.25) | 0.29 | 0.64* |
*P is for interaction of treatment strategy with diabetic status and with time to randomization. The bold entries are related to the 5 years data.
Impact of treatment strategy on mortality in the global population, in patients managed within and after 2 h, in diabetics and non-diabetics according to time of follow-up
| . | Cumulative number of deaths fibrinolysis /angioplasty . | Pre-hospital fibrinolysis (%) . | Primary angioplasty (%) . | Hazard ratio (95% confidence interval) . | P-value . | *P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| 1 month | 16/20 | 3.8 | 4.8 | |||
| 1 year | 23/30 | 5.5 | 7.1 | |||
| 3 years | 31/40 | 7.5 | 9.6 | |||
| 5 years | 40/52 | 9.7 | 12.6 | 0.75 (0.50–1.14) | 0.18 | |
| <2 h | ||||||
| 1 month | 5/13 | 2.2 | 5.7 | |||
| 1 year | 7/17 | 3.0 | 7.4 | |||
| 3 years | 8/21 | 3.5 | 9.2 | |||
| 5 years | 13/25 | 5.8 | 11.1 | 0.50 (0.25–0.98) | 0.04 | |
| >2 h | ||||||
| 1 month | 11/7 | 5.9 | 3.7 | |||
| 1 year | 16/13 | 8.6 | 7.0 | |||
| 3 years | 23/19 | 12.3 | 10.3 | |||
| 5 years | 27/26 | 14.5 | 14.4 | 1.02 (0.59–1.75) | 0.92 | 0.10* |
| Diabetics | ||||||
| 1 month | 6/3 | 13.0 | 5.3 | |||
| 1 year | 8/6 | 17.6 | 10.5 | |||
| 3 years | 8/10 | 17.6 | 17.9 | |||
| 5 years | 9/12 | 20.0 | 21.7 | 0.99 (0.42–2.36) | 0.99 | |
| Non-diabetics | ||||||
| 1 month | 9/14 | 2.4 | 3.9 | |||
| 1 year | 14/21 | 3.8 | 5.8 | |||
| 3 years | 22/27 | 6.0 | 7.5 | |||
| 5 years | 30/37 | 8.2 | 10.5 | 0.77 (0.42–1.25) | 0.29 | 0.64* |
| . | Cumulative number of deaths fibrinolysis /angioplasty . | Pre-hospital fibrinolysis (%) . | Primary angioplasty (%) . | Hazard ratio (95% confidence interval) . | P-value . | *P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| 1 month | 16/20 | 3.8 | 4.8 | |||
| 1 year | 23/30 | 5.5 | 7.1 | |||
| 3 years | 31/40 | 7.5 | 9.6 | |||
| 5 years | 40/52 | 9.7 | 12.6 | 0.75 (0.50–1.14) | 0.18 | |
| <2 h | ||||||
| 1 month | 5/13 | 2.2 | 5.7 | |||
| 1 year | 7/17 | 3.0 | 7.4 | |||
| 3 years | 8/21 | 3.5 | 9.2 | |||
| 5 years | 13/25 | 5.8 | 11.1 | 0.50 (0.25–0.98) | 0.04 | |
| >2 h | ||||||
| 1 month | 11/7 | 5.9 | 3.7 | |||
| 1 year | 16/13 | 8.6 | 7.0 | |||
| 3 years | 23/19 | 12.3 | 10.3 | |||
| 5 years | 27/26 | 14.5 | 14.4 | 1.02 (0.59–1.75) | 0.92 | 0.10* |
| Diabetics | ||||||
| 1 month | 6/3 | 13.0 | 5.3 | |||
| 1 year | 8/6 | 17.6 | 10.5 | |||
| 3 years | 8/10 | 17.6 | 17.9 | |||
| 5 years | 9/12 | 20.0 | 21.7 | 0.99 (0.42–2.36) | 0.99 | |
| Non-diabetics | ||||||
| 1 month | 9/14 | 2.4 | 3.9 | |||
| 1 year | 14/21 | 3.8 | 5.8 | |||
| 3 years | 22/27 | 6.0 | 7.5 | |||
| 5 years | 30/37 | 8.2 | 10.5 | 0.77 (0.42–1.25) | 0.29 | 0.64* |
*P is for interaction of treatment strategy with diabetic status and with time to randomization. The bold entries are related to the 5 years data.
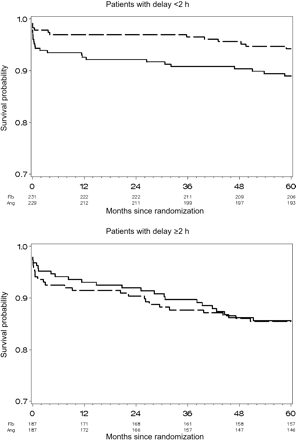
The effect of time to randomization on the outcome of treatment comparison on 5-year mortality is presented in Figure 3.
Mortality as estimated with the Kaplan–Meier method (day of reference for each time interval is the day of randomization) at 5 years (vertical bars), 1 year (dashed bars), and 30 days (black bar) for diabetics (A), non-diabetics (B), patients managed before (C) and after (D) 2 h, and for the overall population (E). The only significant difference at 5 years is for the patients managed within 2 h (C).
In patients included within 2 h of symptom onset, mortality was lower in the pre-hospital fibrinolysis group [5.8% (13 patients) vs. 11.1% (25 patients); RR 0.50; 95% CI 0.25–0.97; P = 0.04], whereas death rates were identical irrespective of the treatment strategies in patients included after 2 h [14.5% (27 patients) vs. 14.4% (26 patients); HR 1.02; 95% CI 0.59–1.75; P = 0.92]. However, interaction between treatment and delay from symptoms did not reach significance (P = 0.10).
In diabetics, the mortality rate was non-significantly higher at 1 year in the pre-hospital fibrinolysis group. However, mortality was similar in both groups at 5 years. There was a definite adjustment in mortality between one and 5 year that write off the strong early trend in favour of primary angioplasty (Figure 4).
Survival according to randomized treatment assignment in patients managed within 2 h and patients managed beyond 2 h. [Pre-hospital fibrinolysis group (broken line) and PCI group (solid line).]. Underneath the graph are the numbers of patients at risk for each time point.
Discussion
The 5-year follow up analysis of the CAPTIM trial confirms the initial trends observed at 30 days. A strategy of pre-hospital fibrinolysis with immediate transfer to an interventional facility for rescue angioplasty if needed appears to yield long-term survival similar to that of primary angioplasty.
Those results do not challenge the general consensus concerning the superiority of timely PCI over in-hospital fibrinolysis.8 The long-term DANAMI2 study confirmed the initial results and has demonstrated the benefit of transferring patients for primary PCI over in-hospital fibrinolysis when patients are initially managed in a community hospital with no rescue angioplasty nor coronary angiography during the hospital stay.5 The early benefit of primary PCI was also sustained during the 5-year follow-up of the PRAGUE-2 study that compared fibrinolysis and transfer to the nearest PCI centre for primary PCI for patients admitted in community hospitals with no in-hospital catheterization facilities.4
In the CAPTIM study, the fibrinolysis strategy was quite different since patients were initially managed by medicalized mobile care unit and immediately transferred to a centre having catheterization facility. This organization contributed to minimize delay to the diagnosis of STEMI and to shorten all delays to treatment, notably timely rescue PCI. In CAPTIM, 70% of patients initially treated with pre-hospital fibrinolysis underwent PCI within the hospital stay either as rescue PCI in those not responding to fibrinolysis or later as elective PCI. So, in this ‘mobile spoke and hub’ organization, the clinical outcome was influenced by the combination of many factors: early fibrinolysis, immediate transportation, and early angiography followed by mechanical reperfusion if needed.
There are, however, differences between populations involved in these three studies. In the CAPTIM study, the 5-year mortality was lower than the 3-year mortality in DANAMI-2 and almost half of the 5-year mortality observed in the PRAGUE 2 study.4,5 This low mortality may be related to population selection. CAPTIM, like most studies in the pre-hospital setting, recruited a younger and lower risk population. Other constitutional or health care factors may influence this long-term favourable survival. In effect, the 1-year mortality in CAPTIM is almost identical to the 1-year mortality of the population-based French nationwide registry Fast-MI that concerned the same population.9
The trend towards a lower mortality in patients treated with the pre-hospital fibrinolytics strategy was already present at the end of the first month and remained stable over the 5-year follow-up, with the same proportional hazard ratio (20%). This trend was in fact driven by the lower mortality in patients managed within 2 h.
For these patients, there was, at 5 years, a significant mortality reduction with the pre-hospital fibrinolysis strategy when compared with primary angioplasty. Their survival curve rapidly separates and remains parallel, indicating that treatment immediately improved survival and that this effect was maintained at long term. This is consistent with the observation that an important part of the initial mortality benefit in the pre-hospital fibrinolysis group was related to a reduction in early cardiogenic shocks.7 This is also in agreement with the hypothesis associated with early fibrinolysis benefit: more effective recanalization and myocardial salvage.10 These benefits are not confined to the CAPTIM study. These benefits have also been observed in the real world population with two important registries that enrolled patients managed with the same pre-hospital strategy.9,11 In the Vienna STEMI registry, there was a trend for a reduced mortality in favour of pre-hospital fibrinolysis in patients managed within 2 h.11 In the FAST-MI registry, mortality at 1 month and 1 year was almost identical to the mortality in CAPTIM in both the fibrinolysis and the primary PCI groups.9
A subanalysis of the CAPTIM study had revealed a non-significant reduction in mortality in diabetics with the PCI strategy.12 This observation tallies with the 50% reduction in early mortality observed in a pooled analysis of individual patient's data, when primary PCI was compared with in-hospital fibrinolysis in diabetics.13 Whether this important clinical benefit of primary PCI is sustained with time seems now unlikely. Our results are in agreement with the subgroup analysis of diabetics in the DANAMI2 trial.14 At 3 years follow-up, mortality was similar in the primary PCI and in-hospital fibrinolysis groups. In the DANAMI2 diabetics subgroup, there was even a trend towards a higher re-infarction rate in the primary PCI group. Therefore, compared with fibrinolysis, PCI in diabetics appears to reduce early morality but does not bring substantial benefit on long-term mortality. The operating factors are probably intrinsic to diabetes.
Limitations
As for any long-term and subgroup analysis, caution is required in interpreting the present data. A total of 45 patients lost to follow up. Most of those patients had changed their address and were not born in France making the assessment of vital status through registries of patients’ birthplace inoperative. Owing to the small number of patients lost from follow-up, it is unlikely that this limitation has a substantial influence on the trends or differences in mortality. We did not collect information about the other components of the main composite endpoint of the CAPTIM study (re-infarction and stroke) nor about medications. These data would have been difficult to ascertain, their absence limits the interpretation of the long-term mortality results. Overall, the sample size is limited especially for the subgroup analysis on delay and diabetics. P-values should be interpreted with extreme caution and should be viewed as hypothesis generating, given that these analyses were not pre-specified and the multiplicity of analyses was not planned. Even if the results of the present analysis complete and enrich the previous subgroup analyses on the CAPTIM study population, this analysis should be considered as hypothesis-generating analysis.
Conclusion
This long-term follow-up of the CAPTIM study confirms that in reference to mortality, primary PCI does not bring benefit compared with a strategy of pre-hospital fibrinolysis with immediate transfer for rescue angioplasty if needed, in patients with an acute ST-segment elevation myocardial infarction managed within 6 h of the symptoms onset. However, in patients managed within 2 h of symptom onset, the pre-hospital fibrinolysis strategy reduced long-term mortality. These data underline that different reperfusion strategies might bring similar results at the acute phase of a myocardial infarction when an appropriate pre-hospital organization is operative.
Funding
This study was supported by a grant from the French Ministry of Health (Projet Hospitalier de Recherche Clinique, 96/045), by the Hospices Civils de Lyon, and a research grant from AstraZeneca France. Biotronik Gmbh provided balloons and guidewires free of charge. The 5-year analysis was in part supported by a grant form Boehringer Ingelheim.
Conflict of interest: none declared.
Appendix: The CAPTIM Investigators
Executive committee: P. Touboul, E.B., A. Leizorovicz, J.-M. Lacroute, J. Cassagnes, and J.L. Blanc.
Steering committee: P. Touboul (Study Chairman), A. Leizorovicz (Clinical Director, Coordinating Centre), and E.B. (Director, Intermediate Coordinating Centre).
Coordinating centre: P. Touboul, E.B., A. Leizorovicz, J.P. Teppe, C. Fernandez, V. Bost, A. Akkal, Z. Akkal, C. Thien, F. Bugnard, C. Mercier, S. Chabaud, F. Lorenzelli, N. Visele, J.-M. Lacroute, and J. Cassagnes.
Cardiology: J. Beaune (Lyon); J. Cassagnes (Clermont-Ferrand); S. Cattan (Le Raincy); N. Danchin (Vandoeuvre-les-Nancy); J.L. Dubois-Rande, P. Dupouy (Créteil); G. Grollier (Caen); K. Isaaz (Saint-Etienne); T. Jullien (Saint-Denis); G.K. (Lyon); J. Machecourt (Grenoble); J. Puel (Toulouse); J.C. Quiret (Amiens); M. Slama (Clamart); C. Spaulding, and P.G.S. (Paris).
SAMU: J.L. Blanc (Saint-Etienne); F. Boudet, E. Boullenger (Toulouse); C. Carmes (Caen); P. Cristofini (Paris); F.D. (Clermont-Ferrand); J.-M. Lacroute (Grenoble); F.L., P. Magne (Bobigny); A. Margenet (Créteil); L. Nace (Nancy); B. Nemitz (Amiens); J. Pasteyer (Garches); G. Prost (Lyon).
Writing committee: E.B., A. Leizorovicz, F.L., C. Mercier, E.P. McFadden, P.G.S., and P. Touboul.
Safety and efficacy monitoring committee: J.P. Bassand (cardiologist), B. Charbonnier (cardiologist), P. Goldstein (emergency physician), R. Grolleau (cardiologist), Y. Lambert (emergency physician).
Critical events committee: B. Coppere (Lyon); L. Holzapfel (Bourg-en-Bresse); D. Jacques, M. Lopez, N. Nighoghossian (Lyon); L. Ollivier (Vienne); B. De Breyne (Lyon).
Mobile intensive care units (SAMU): SAMU 93, Bogigny (F.L., C. Lapandry, P. Magne); SAMU 69, Lyon (G. Prost, P.Y.D., E. Grand, P. Petit); SAMU 75, Paris (P. Cristofini); SAMU 31, Toulouse (F. Boudet, W. Saidi, E. Boullenger, S. Charpentier); SAMU 38, Grenoble (J.-M. Lacroute); SAMU 63, Clermont-Ferrand (F.D., Ch. Lespiaucq); SAMU 80, Amiens (B. Nemitz, C. Boyer); SAMU 94, Créteil (A. Margenet, C. Bertrand); SAMU 92, Garches (J. Pasteyer, F. Templier); SAMU 14, Caen (C. Carmes); SAMU 42, Saint-Etienne (J.L. Blanc); SAMU 54, Nancy (L. Nace).
Participating hospitals (Departments of Cardiology): Lyon (J. Beaune, E.B., G. Durand De Gevigney, G. Finet, M. Fatemi, G. Hadour, G.K.); Le Raincy (S. Cattan); Grenoble (J. Machecourt, G.V., S. Richard); Clermont-Ferrand (J. Cassagnes, J.R. Lusson, P. Motreff, D. Lusson); St-Denis (T. Jullien, F. Drane); Amiens (J.C. Quiret, L. Leborgne, G. Jarry); Créteil (J.L. Dubois-Rande, S. El Hamine, P. Dupouy, E. Teiger, F. Veyssière); Toulouse, Hôpital Purpan (J. Puel, M. Jean, M. Elbaz); Toulouse, Hôpital Rangueil (M. Galinier, J.R., P. Cabrol); Montreuil-Sous-Bois (R. Gryman, A. Boccara, V. Fourchard, A. Kadiri); Paris, Hôpital Lariboisière (P. Coumel, P. Beaufils, F. Tarragano); Boulogne-Billancourt (J.P. Bourdarias, T. Joseph); Paris, Hôpital Cochin (C. Spaulding, P. Richard, F. Belaouchi); Caen (G. Grollier, S. Gibert); Paris, Hôpital Bichat (P.G.S., P. Aubry, R. Farnoud, K. Picauville, E. Colloc); Paris, Hôpital Tenon (A. Vahanian, E. Garbaz, P. Michaud); Saint-Etienne (K. Isaaz, A. Cerisier, L. Richard); Paris, Hôpital Necker (J.P. Metzger, F. Beygui); Paris, Hôpital Boucicaut (C. Guerot, K. Bougrini, E. Durand); Clamart (M. Slama, Y. Carel, P. Colin); Nancy (E. Aliot, G. Ethevenot, R. Krafft); Paris, Hôpital du Val de Grâce (J. Monsegu); Vandoeuvre les Nancy (N. Danchin, P. Houriez); Neuilly/Seine (S. Makowski, R. Pilliere); Paris, Clinique Bizet (P. Durand, R. Cador, M. Lesueur); Paris, Hôpital P Salpétrière (G. Drobinski, D. Thomas); Suresnes (M. Francoual).
References
Author notes
A complete list of CAPTIM investigators and personnel can be found in the appendix and in Busk et al.5



![Survival according to randomized treatment assignment. [Pre-hospital fibrinolysis group (broken line) and PCI group (solid line).] Underneath the graph are the numbers of patients at risk for each time point (those who survived and had been at least followed up to this point).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/30/13/10.1093/eurheartj/ehp156/2/m_ehp15601.gif?Expires=1716321161&Signature=P5fHI1vo4i99kcv3wM1017l9dwkKSVTUCFVYAxY9ACRUhxFEsnwYwWtCj-kRKA39elhas89BsVdo1hp-gNWXQ7QFvTmx4hKkxGCQJLeVKnXC7DCxr6Mcvjf6yUavfSW10zth5q~WuLyLdcyxF8GRjWdYfQQCU1YHPw2jkdZCYgdOF8KDT1sMWl6maQn0K9cr2zGNDi5CKqKqeiKhoTtJq3g-nGlDuppCllX6MT~1pW~VGJK5nfHwiGRp5Z1nziBKPexd2VxC39VQvQOV2uqEnSlaKzb6aFttVGgAvHEmPU6nHD6c8WsxRXJCOPsfAZOq-vemvP-47TeZME0HCdBOZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Survival according to randomized treatment assignment in diabetics and non-diabetics. [Pre-hospital fibrinolysis group (broken line) and PCI group (solid line).] Underneath the graph are the numbers of patients at risk for each time point.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/30/13/10.1093/eurheartj/ehp156/2/m_ehp15602.gif?Expires=1716321161&Signature=Py8sAaBB8PscERNXc5PayjXt4ZZLhVYbQ0Myw5l4XgFlbfxWFJ~3nCKJQmOXfdTkZA1LgdOufbty-oIsnnKSjLKhA1QZRTC3DAMxI~FR-PPSUS8zndsims0OcR3oBgCZ~e0rWuJ6eMq0iDWna5OmepvYIczXCSUl0TB8Ee0Bul7CJYvKjISuV3ivS~YeueXjQU-RxwthxrTVKIOWewuWfzKAoEmWwoFe1krGjdX2aNu3lsU7-uhIS4f-IQ4xxj2z~SnFZU2vvapXzPH8O-eHrCLhbgmrCmaw9HCtrUjeWt1m~QM9YzlcmU2fAMyM7q3jZbqyfFNFKQt0ZJt8VbFzGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Survival according to randomized treatment assignment in patients managed within 2 h and patients managed beyond 2 h. [Pre-hospital fibrinolysis group (broken line) and PCI group (solid line).]. Underneath the graph are the numbers of patients at risk for each time point.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/30/13/10.1093/eurheartj/ehp156/2/m_ehp15604.gif?Expires=1716321161&Signature=2CKUZJkXO1VECrM6QfiShkUIn6AkT6ShxDTeXvcX6umWmQzVjL3c3zPh9gYfqayMfxlUUA5etMZ5AgaRtCFpJimHjdUdcVO9RixbnMcurqxIR~1WnmqoVqDUql0XydJvDUfdQPiw6acgim9QB3I1nZivfHRGWq9zvtqXgDMPZea8JwYLmrSxYcrTSt8Wsm478vjeYDasqBrr37S3ljZvJweSJEpUmc7ALdYML153aqhAlMEkFoLAtXcRY-MG5S33GTilyHq6Ebikd5lZW6X35Tb0015lgmb3z9AI4CqHYCDxlJkr0EUHNFomhSRWZ6cp1Xra99PXba6PJxoTl1dVqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
