-
PDF
- Split View
-
Views
-
Cite
Cite
Frédéric Barbey, Salah D. Qanadli, Christoph Juli, Noureddine Brakch, Tomáš Palaček, Elena Rizzo, Xavier Jeanrenaud, Boris Eckhardt, Aleš Linhart, Aortic remodelling in Fabry disease, European Heart Journal, Volume 31, Issue 3, February 2010, Pages 347–353, https://doi.org/10.1093/eurheartj/ehp426
Close - Share Icon Share
Abstract
To evaluate thoracic aortic dilation in patients with Fabry disease (FD).
A cohort of 106 patients with FD (52 males; 54 females) from three European centres were studied. The diameter of the thoracic aorta was assessed at three levels (sinus of Valsalva, ascending aorta, and descending aorta) using echocardiograms and cardiovascular magnetic resonance imaging. Aortic dilation at the sinus of Valsalva was found in 32.7% of males and 5.6% of females; aneurysms were present in 9.6% of males and 1.9% of females. No aortic dilation was observed in the descending aorta. There was no correlation between aortic diameter at the sinus of Valsalva and cardiovascular risk factors.
Fabry disease should be considered as a cardiovascular disease that affects the heart and arterial vasculature, including the thoracic aorta. Thus, patients with FD should be closely monitored for the presence, and possible progression and complications of aortic dilation.
Clinical Trial Registration: Protocol 101/01. Ethics committee, Faculty of Medicine, Lausanne.
Introduction
Fabry disease (FD) is a rare X-linked lysosomal storage disorder (LSD) caused by deficiency of the enzyme α-galactosidase A. This deficiency results in the progressive intracellular deposition of glycosphingolipids (primarily globotriaosylceramide), particularly in vascular endothelial and smooth muscle cells, the myocardium, renal epithelium, and dorsal root ganglion cells.1,2 The condition is associated with multisystemic manifestations, including left ventricular hypertrophy (LVH), renal failure, and stroke, and may induce premature death as a result of progressive cardiac, renal, and central nervous system involvement.1,3,4 Although FD has traditionally been classified as an LSD, increasing evidence suggests that the disease should also be regarded as a cardiovascular disease, affecting the myocardium and arterial vasculature.
The most frequent cardiac finding in FD is a high prevalence of LVH, usually concentric, which typically develops during or after the third decade of life.5 Patients with Fabry cardiomyopathy ultimately develop progressive heart failure, mostly due to impaired LV filling.6 Vascular remodelling has been a consistent finding in small- and medium-sized arteries in FD. Marked and accelerated increases in intima–media thickness (IMT) of the radial artery and the common carotid artery (CCA) have been reported.7,8
Other studies looking at cardiac parameters in small groups of patients have suggested changes at the level of the thoracic aorta. Several such studies reported increased aortic root diameter in males with FD relative to females with FD and historical healthy controls.9–12 In addition, dilation and thickening of the thoracic aorta have been described in a few post-mortem reports.2,13,14 However, it is not clear whether aortic remodelling is a characteristic feature of FD. The aim of the present study was to assess structural changes in the aorta in a large cohort of patients with FD. We hypothesized that FD may predispose to remodelling of the aorta, which could lead to aortic dilation.
Methods
This observational study population consisted of 112 consecutive adults with FD referred to three specialized centres for complete clinical assessments. Index cases were referred because of acroparaesthesia, angiokeratomas, cornea verticillata, or renal failure. Genetic screening of related family members was undertaken following the identification of each index case. Informed consent was obtained from all patients. The Institutional Review Boards for each centre approved the study.
Patient population
Between 1 October 2000 and 31 December 2005, a total of 112 consecutively referred adults with confirmed FD from 34 different families were evaluated. All patients were 18 years of age or older, no females were pregnant and no patient was receiving enzyme replacement therapy at the time of baseline evaluation. All males presented with the classic form of FD, which was confirmed by the detection of α-galactosidase A deficiency in leukocytes and plasma.15 In females, diagnosis was confirmed by genetic analysis.
All patients underwent a baseline assessment that included physical examination, blood pressure measurement, laboratory blood tests, and measurements of the thoracic aorta. Measurements of aortic diameter varied between the three centres. Sixty-six patients underwent transthoracic echocardiography (TTE), 36 patients underwent TTE and cardiovascular magnetic resonance imaging (MRI), and 10 were assessed using cardiovascular MRI only. Echocardiographic data from six patients could not be analysed because of poor cardiac or aortic echogenicity. These patients were excluded from the analysis. Thus, 106 patients were eligible for the study.
Procedures and measurements
Transthoracic echocardiography was used to evaluate aortic diameter at the sinus of Valsalva, interventricular septum (IVS) thickness, and aortic valve integrity. M-mode and two-dimensional echocardiography were performed by three experienced ultrasonographers, using commercially available equipment (Acuson Sequoia, Siemens, Forchheim, Germany, and Toshiba Powervision) with a broadband phased-array transducer. Measurements were taken in triplicate according to the American Society of Echocardiography recommendations.16 Offline, two-dimensional measurements of the aortic root at the sinus of Valsalva were made at end-diastole. Measurements of the ascending aorta were performed 2 cm above the sino-tubular junction by M-mode echocardiography. The aortic valve was examined with M-mode and two-dimensional echocardiography for the presence of structural abnormalities and using colour Doppler imaging for the presence of functional abnormalities. A four-degree classification was used to quantify aortic regurgitation.17
Cardiovascular MRI was performed using a 1.5-T Philips MRI scanner (Philips Medical Systems, Amsterdam, the Netherlands) or 1.5-T Symphony scanner (Siemens) using a phase-array cardiac coil. In order to measure aortic diameters, two sequences were obtained: transverse single-phase ECG-gated (end-diastolic) breath-hold images of the thorax with a steady-state free precession (SSFP) sequence (6 mm slice thickness), and retrospective ECG-gated breath-hold short-axis cine images (25 frames per cardiac cycle) of the LV outflow tract (SSFP turbo-field echo sequence). Three experienced radiologists analysed images on a workstation using commercially available software to measure the diameters of the sinus of Valsalva (at the same level as assessed using TTE), ascending aorta, and descending aorta.18
Statistical analysis
All statistical analyses were performed using S-PLUS® 6.2 for Windows Professional Edition. The relationships between relevant variables [gender, age, body surface area (BSA), body mass index (BMI), and blood pressure] and diameters were assessed using multiple regression analyses. A value of P < 0.05 was considered statistically significant. The dimensions used as reference criteria to define normal and dilated aortic diameters, aneurysms, and normal IVS thickness are shown in Table 1.18
| Anatomic measurement . | Dimension (mm) . | |
|---|---|---|
| . | Female . | Male . |
| Sinus of Valsalva | ||
| Normal diameter | 30 ± 3 | 34 ± 3 |
| Dilation | >36 | >40 |
| Aneurysm | >1.5 × predicted valuea | >1.5 × predicted valuea |
| Ascending aorta | ||
| Normal diameter | 26 ± 3 | 29 ± 3 |
| Dilation | >32 | >35 |
| Descending aorta | ||
| Dilation | >28 | >28 |
| Abnormal interventricular septum thickness | >10 | >11 |
| Anatomic measurement . | Dimension (mm) . | |
|---|---|---|
| . | Female . | Male . |
| Sinus of Valsalva | ||
| Normal diameter | 30 ± 3 | 34 ± 3 |
| Dilation | >36 | >40 |
| Aneurysm | >1.5 × predicted valuea | >1.5 × predicted valuea |
| Ascending aorta | ||
| Normal diameter | 26 ± 3 | 29 ± 3 |
| Dilation | >32 | >35 |
| Descending aorta | ||
| Dilation | >28 | >28 |
| Abnormal interventricular septum thickness | >10 | >11 |
aPredicted value for the upper-normal limit for aortic diameter at the sinuses of Valsalva, in both men and women, was computed as 21 mm/m2 × body surface area.
| Anatomic measurement . | Dimension (mm) . | |
|---|---|---|
| . | Female . | Male . |
| Sinus of Valsalva | ||
| Normal diameter | 30 ± 3 | 34 ± 3 |
| Dilation | >36 | >40 |
| Aneurysm | >1.5 × predicted valuea | >1.5 × predicted valuea |
| Ascending aorta | ||
| Normal diameter | 26 ± 3 | 29 ± 3 |
| Dilation | >32 | >35 |
| Descending aorta | ||
| Dilation | >28 | >28 |
| Abnormal interventricular septum thickness | >10 | >11 |
| Anatomic measurement . | Dimension (mm) . | |
|---|---|---|
| . | Female . | Male . |
| Sinus of Valsalva | ||
| Normal diameter | 30 ± 3 | 34 ± 3 |
| Dilation | >36 | >40 |
| Aneurysm | >1.5 × predicted valuea | >1.5 × predicted valuea |
| Ascending aorta | ||
| Normal diameter | 26 ± 3 | 29 ± 3 |
| Dilation | >32 | >35 |
| Descending aorta | ||
| Dilation | >28 | >28 |
| Abnormal interventricular septum thickness | >10 | >11 |
aPredicted value for the upper-normal limit for aortic diameter at the sinuses of Valsalva, in both men and women, was computed as 21 mm/m2 × body surface area.
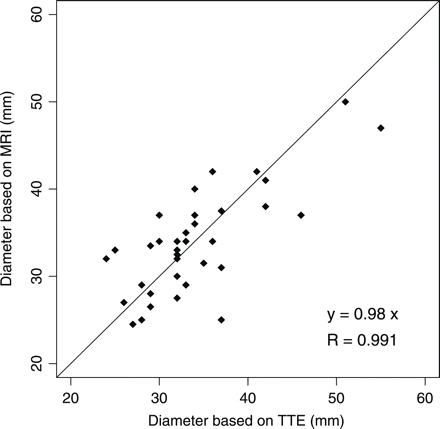
A paired student's t-test was used to determine whether TTE and MRI measurements of aortic diameter at the sinus of Valsalva could be pooled in order to obtain results with higher statistical power. The student's t-test was performed on 36 pairs of measurements. The resulting P-value of 0.814 indicates that the difference between TTE and MRI measurements is statistically not significant. The 36 pairs of measurements that are displayed on Figure 1 confirm that TTE and MRI results are comparable. For patients with both TTE and MRI measurements, the mean of the measurements was used in the analyses, unless otherwise indicated.
Magnetic resonance imaging (MRI) and transthoracic echocardiography (TTE) measurements of aortic diameter at the sinus of Valsalva for 36 patients with Fabry disease. The student t-test value (P-value = 0.814) confirms that TTE and MRI results are comparable.
Statement of responsibility
The authors had full access to all data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline clinical data are summarized in Table 2. All patients were Caucasian. In comparison with female patients, males had a lower BMI and lower levels of total serum, low-density lipoprotein and high-density lipoprotein cholesterol, a greater BSA and IVS thickness, and a higher serum creatinine level; these differences were statistically significant. Two males were on dialysis and four males had functioning kidney grafts. The ejection fraction of the LV was ≥50% in all patients, thus excluding the presence of significant LV systolic insufficiency (no case of LV dilation).
Baseline clinical characteristics of the analysed population
| Parameters . | Males (n = 52)a . | Females (n = 54)a . | P-value . |
|---|---|---|---|
| Age (years) | 39.9 ± 11.7 | 43.4 ± 17.9 | 0.236 |
| Height (cm) | 173.6 ± 7.2 | 162.7 ± 5.6 | <0.001 |
| Weight (kg) | 67.3 ± 10.8 | 63.4 ± 10.9 | 0.069 |
| Body surface area (m2) | 1.8 ± 0.16 | 1.67 ± 0.13 | <0.001 |
| Body mass index (kg/m2) | 22.3 ± 3 | 24 ± 4.5 | 0.019 |
| Systolic blood pressure (mmHg) | 123 ± 12.2 | 125 ± 16.9 | 0.485 |
| Diastolic blood pressure (mmHg) | 76.8 ± 7.9 | 76.5 ± 9.6 | 0.845 |
| Serum total cholesterol (mmol/L) | 4.73 ± 1.05 | 5.5 ± 1.1 | <0.001 |
| Serum HDL cholesterol (mmol/L) | 1.43 ± 0.34 | 1.63 ± 0.43 | 0.01 |
| Serum LDL cholesterol (mmol/L) | 2.72 ± 0.83 | 3.29 ± 1.02 | 0.003 |
| Ratio of total to HDL cholesterol | 3.45 ± 0.98 | 3.57 ± 1.07 | 0.529 |
| Serum triglycerides (mmol/L) | 1.22 ± 0.62 | 1.27 ± 0.53 | 0.662 |
| Serum creatinine (µmol/L) | 125.1 ± 74.2 | 81.2 ± 17 | <0.001 |
| Patients on dialysis | 2 | 0 | |
| Patients with kidney graft | 4 | 0 | |
| Interventricular septum (mm) | 15.3 ± 4.3 | 12.2 ± 4.6 | 0.001 |
| Left ventricular ejection fraction (%) | 64.5 ± 7.4 | 66.1 ± 6.9 | 0.263 |
| Current smokers, n (%) | 9 (17) | 5 (9) | 0.349 |
| Antihypertensive treatment, n (%) | 17 (33) | 13 (24) | 0.442 |
| Statin therapy | 17 (33) | 16 (29) | 0.422 |
| Diabetes [n (%)] | 0 (0) | 2 (4) | 0.492 |
| Parameters . | Males (n = 52)a . | Females (n = 54)a . | P-value . |
|---|---|---|---|
| Age (years) | 39.9 ± 11.7 | 43.4 ± 17.9 | 0.236 |
| Height (cm) | 173.6 ± 7.2 | 162.7 ± 5.6 | <0.001 |
| Weight (kg) | 67.3 ± 10.8 | 63.4 ± 10.9 | 0.069 |
| Body surface area (m2) | 1.8 ± 0.16 | 1.67 ± 0.13 | <0.001 |
| Body mass index (kg/m2) | 22.3 ± 3 | 24 ± 4.5 | 0.019 |
| Systolic blood pressure (mmHg) | 123 ± 12.2 | 125 ± 16.9 | 0.485 |
| Diastolic blood pressure (mmHg) | 76.8 ± 7.9 | 76.5 ± 9.6 | 0.845 |
| Serum total cholesterol (mmol/L) | 4.73 ± 1.05 | 5.5 ± 1.1 | <0.001 |
| Serum HDL cholesterol (mmol/L) | 1.43 ± 0.34 | 1.63 ± 0.43 | 0.01 |
| Serum LDL cholesterol (mmol/L) | 2.72 ± 0.83 | 3.29 ± 1.02 | 0.003 |
| Ratio of total to HDL cholesterol | 3.45 ± 0.98 | 3.57 ± 1.07 | 0.529 |
| Serum triglycerides (mmol/L) | 1.22 ± 0.62 | 1.27 ± 0.53 | 0.662 |
| Serum creatinine (µmol/L) | 125.1 ± 74.2 | 81.2 ± 17 | <0.001 |
| Patients on dialysis | 2 | 0 | |
| Patients with kidney graft | 4 | 0 | |
| Interventricular septum (mm) | 15.3 ± 4.3 | 12.2 ± 4.6 | 0.001 |
| Left ventricular ejection fraction (%) | 64.5 ± 7.4 | 66.1 ± 6.9 | 0.263 |
| Current smokers, n (%) | 9 (17) | 5 (9) | 0.349 |
| Antihypertensive treatment, n (%) | 17 (33) | 13 (24) | 0.442 |
| Statin therapy | 17 (33) | 16 (29) | 0.422 |
| Diabetes [n (%)] | 0 (0) | 2 (4) | 0.492 |
aValues expressed as mean ± SD unless otherwise indicated. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Baseline clinical characteristics of the analysed population
| Parameters . | Males (n = 52)a . | Females (n = 54)a . | P-value . |
|---|---|---|---|
| Age (years) | 39.9 ± 11.7 | 43.4 ± 17.9 | 0.236 |
| Height (cm) | 173.6 ± 7.2 | 162.7 ± 5.6 | <0.001 |
| Weight (kg) | 67.3 ± 10.8 | 63.4 ± 10.9 | 0.069 |
| Body surface area (m2) | 1.8 ± 0.16 | 1.67 ± 0.13 | <0.001 |
| Body mass index (kg/m2) | 22.3 ± 3 | 24 ± 4.5 | 0.019 |
| Systolic blood pressure (mmHg) | 123 ± 12.2 | 125 ± 16.9 | 0.485 |
| Diastolic blood pressure (mmHg) | 76.8 ± 7.9 | 76.5 ± 9.6 | 0.845 |
| Serum total cholesterol (mmol/L) | 4.73 ± 1.05 | 5.5 ± 1.1 | <0.001 |
| Serum HDL cholesterol (mmol/L) | 1.43 ± 0.34 | 1.63 ± 0.43 | 0.01 |
| Serum LDL cholesterol (mmol/L) | 2.72 ± 0.83 | 3.29 ± 1.02 | 0.003 |
| Ratio of total to HDL cholesterol | 3.45 ± 0.98 | 3.57 ± 1.07 | 0.529 |
| Serum triglycerides (mmol/L) | 1.22 ± 0.62 | 1.27 ± 0.53 | 0.662 |
| Serum creatinine (µmol/L) | 125.1 ± 74.2 | 81.2 ± 17 | <0.001 |
| Patients on dialysis | 2 | 0 | |
| Patients with kidney graft | 4 | 0 | |
| Interventricular septum (mm) | 15.3 ± 4.3 | 12.2 ± 4.6 | 0.001 |
| Left ventricular ejection fraction (%) | 64.5 ± 7.4 | 66.1 ± 6.9 | 0.263 |
| Current smokers, n (%) | 9 (17) | 5 (9) | 0.349 |
| Antihypertensive treatment, n (%) | 17 (33) | 13 (24) | 0.442 |
| Statin therapy | 17 (33) | 16 (29) | 0.422 |
| Diabetes [n (%)] | 0 (0) | 2 (4) | 0.492 |
| Parameters . | Males (n = 52)a . | Females (n = 54)a . | P-value . |
|---|---|---|---|
| Age (years) | 39.9 ± 11.7 | 43.4 ± 17.9 | 0.236 |
| Height (cm) | 173.6 ± 7.2 | 162.7 ± 5.6 | <0.001 |
| Weight (kg) | 67.3 ± 10.8 | 63.4 ± 10.9 | 0.069 |
| Body surface area (m2) | 1.8 ± 0.16 | 1.67 ± 0.13 | <0.001 |
| Body mass index (kg/m2) | 22.3 ± 3 | 24 ± 4.5 | 0.019 |
| Systolic blood pressure (mmHg) | 123 ± 12.2 | 125 ± 16.9 | 0.485 |
| Diastolic blood pressure (mmHg) | 76.8 ± 7.9 | 76.5 ± 9.6 | 0.845 |
| Serum total cholesterol (mmol/L) | 4.73 ± 1.05 | 5.5 ± 1.1 | <0.001 |
| Serum HDL cholesterol (mmol/L) | 1.43 ± 0.34 | 1.63 ± 0.43 | 0.01 |
| Serum LDL cholesterol (mmol/L) | 2.72 ± 0.83 | 3.29 ± 1.02 | 0.003 |
| Ratio of total to HDL cholesterol | 3.45 ± 0.98 | 3.57 ± 1.07 | 0.529 |
| Serum triglycerides (mmol/L) | 1.22 ± 0.62 | 1.27 ± 0.53 | 0.662 |
| Serum creatinine (µmol/L) | 125.1 ± 74.2 | 81.2 ± 17 | <0.001 |
| Patients on dialysis | 2 | 0 | |
| Patients with kidney graft | 4 | 0 | |
| Interventricular septum (mm) | 15.3 ± 4.3 | 12.2 ± 4.6 | 0.001 |
| Left ventricular ejection fraction (%) | 64.5 ± 7.4 | 66.1 ± 6.9 | 0.263 |
| Current smokers, n (%) | 9 (17) | 5 (9) | 0.349 |
| Antihypertensive treatment, n (%) | 17 (33) | 13 (24) | 0.442 |
| Statin therapy | 17 (33) | 16 (29) | 0.422 |
| Diabetes [n (%)] | 0 (0) | 2 (4) | 0.492 |
aValues expressed as mean ± SD unless otherwise indicated. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
At baseline, male and female patients had comparable mean systolic and diastolic arterial blood pressures, and all patients had blood pressure within normal range (with or without antihypertensive treatment). Seventeen male (33%) and 13 female (24%) patients were under antihypertensive therapy: one drug in 20 patients (11 males/9 females), two drugs in 8 (4 males/4 females), and three drugs in 2 male patients. Antihypertensive treatment included β-blockers (n = 12; 5 males/7 females), ACEI/ARB (n = 16; 13 males/3 females), diuretics (n = 6; 3 males/3 females), and calcium channel blockers (n = 8; 4 males/4 females). All other baseline parameters were also comparable between the genders.
Minor structural changes of the aortic valve (grade 1) were found in 21 males (40%) and 23 females (43%). One male patient (aged 45 years) had undergone an aortic valve replacement 5 years previously. This patient had dilation at the sinus of Valsalva. The aortic valve was bicuspid in one female patient (aged 27 years); she had a normal aortic diameter.
Abnormal IVS thickening (Table 1) was present in 42 males (80%) and 31 females (57%).
Thoracic aortic dilation was present at the sinus of Valsalva and ascending aorta in 32.7% and 29.6% of male patients, respectively. For male patients, the mean diameters of the thoracic aorta at the sinus of Valsalva and ascending aorta (Table 3) were significantly greater than normal (Table 1) (P < 0.001 and P = 0.004, respectively). In females, thoracic dilation at the sinus of Valsalva and ascending aorta was present in 5.6% (3 of 54 patients) and 21.1% (4 of 19 patients) of patients, respectively. However, for female patients, the mean diameters at these two levels of the aorta were within the normal range (P = 0.341 and P = 0.209, respectively). Dilation at the descending aorta was not observed in any patient.
Thoracic aorta diameters by gender
| . | Females . | Males . | ||||
|---|---|---|---|---|---|---|
| . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . |
| Sinus of Valsalvaa | 54 | 30.8 ± 4.8 (23–51) | 3 (5.6) | 52 | 37.9 ± 5.9 (28–55) | 17 (32.7) |
| Ascending aortab | 19 | 27.1 ± 4.2 (20–34) | 4 (21.1) | 27 | 32.3 ± 5.5 (23–43) | 8 (29.6) |
| Descending aortab | 19 | 19.4 ± 1.7 (16.5–22) | 0 | 27 | 21.4 ± 2.8 (14–27) | 0 |
| . | Females . | Males . | ||||
|---|---|---|---|---|---|---|
| . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . |
| Sinus of Valsalvaa | 54 | 30.8 ± 4.8 (23–51) | 3 (5.6) | 52 | 37.9 ± 5.9 (28–55) | 17 (32.7) |
| Ascending aortab | 19 | 27.1 ± 4.2 (20–34) | 4 (21.1) | 27 | 32.3 ± 5.5 (23–43) | 8 (29.6) |
| Descending aortab | 19 | 19.4 ± 1.7 (16.5–22) | 0 | 27 | 21.4 ± 2.8 (14–27) | 0 |
SD, standard deviation.
aDiameters measured by transthoracic echocardiography (TTE) and/or cardiac magnetic resonance imaging (MRI); the mean of TTE and MRI was used for 36 patients (21 males) in whom both measurements were available.
bDiameter measurements for the ascending and descending aorta are available only for 46 patients who underwent cardiac MRI.
Thoracic aorta diameters by gender
| . | Females . | Males . | ||||
|---|---|---|---|---|---|---|
| . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . |
| Sinus of Valsalvaa | 54 | 30.8 ± 4.8 (23–51) | 3 (5.6) | 52 | 37.9 ± 5.9 (28–55) | 17 (32.7) |
| Ascending aortab | 19 | 27.1 ± 4.2 (20–34) | 4 (21.1) | 27 | 32.3 ± 5.5 (23–43) | 8 (29.6) |
| Descending aortab | 19 | 19.4 ± 1.7 (16.5–22) | 0 | 27 | 21.4 ± 2.8 (14–27) | 0 |
| . | Females . | Males . | ||||
|---|---|---|---|---|---|---|
| . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . | N . | Mean ± SD (mm) (Range) . | Number of patients with aortic dilation [n (%)] . |
| Sinus of Valsalvaa | 54 | 30.8 ± 4.8 (23–51) | 3 (5.6) | 52 | 37.9 ± 5.9 (28–55) | 17 (32.7) |
| Ascending aortab | 19 | 27.1 ± 4.2 (20–34) | 4 (21.1) | 27 | 32.3 ± 5.5 (23–43) | 8 (29.6) |
| Descending aortab | 19 | 19.4 ± 1.7 (16.5–22) | 0 | 27 | 21.4 ± 2.8 (14–27) | 0 |
SD, standard deviation.
aDiameters measured by transthoracic echocardiography (TTE) and/or cardiac magnetic resonance imaging (MRI); the mean of TTE and MRI was used for 36 patients (21 males) in whom both measurements were available.
bDiameter measurements for the ascending and descending aorta are available only for 46 patients who underwent cardiac MRI.
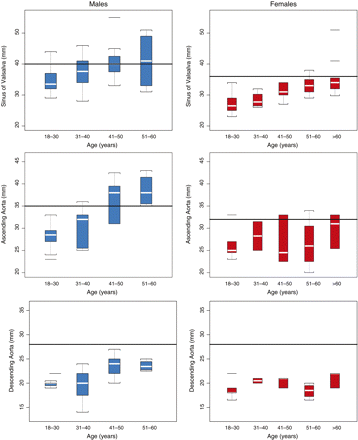
Thoracic aortic dilation appeared in male patients at a significantly earlier age than in females (Figure 2). The mean age of patients with dilation at the sinus of Valsalva was 46.8 years (range, 25–60 years) for males and 69.1 years (range, 55–80 years) for females (P = 0.001). In male patients who were ≥40 years of age, 35.7% had some degree of aortic dilation at the sinus of Valsalva; this proportion rose to 55% in males aged ≥50 years. Aortic dilation was not observed in females <50 years.
Box plot of aortic diameters at three thoracic levels (sinus of Valsalva, ascending aorta, and descending aorta) by age and gender. Box plots include the median (white horizontal line), interquartile range (boxed area), and maximum and minimum scores. Outliers are marked by a short horizontal line. The horizontal rule in each graph indicates the threshold of dilation for each aortic level and gender (Table 1).
In males, dilation was equally prevalent at the sinus of Valsalva (17 of 52 patients; 32.7%) and ascending aorta (8 of 27; 29.6%) (Table 3). Concomitant dilations at the sinus of Valsalva and ascending aorta were observed in five male patients; their age (mean, 48.8 years) was comparable to that of males with dilation restricted to the sinus of Valsalva (mean, 46.0 years) (P = 0.441). A total of six patients [5 of 52 males (9.6%) and 1 of 54 females (1.9%)] had aneurysms at the sinus of Valsalva. In these cases, male patients (mean age, 49.6 years) were younger than the female (80 years of age), a pattern similar to that seen for aortic dilation. The baseline clinical characteristics of these five male patients were comparable to those of the male patients without aneurysm, except for IVS thickness which was significantly increased (P < 0.001). Cardiac MRI data available for two male patients indicated that both had concomitant dilation of the ascending aorta.
A multivariate regression analysis was performed to identify factors that might be linked to aortic dilation at the sinus of Valsalva (Table 4). Gender (P < 0.0001), age (P = 0.001), and IVS thickness (P < 0.0001) were strongly associated with dilation of the sinus of Valsalva. There was no association with serum creatinine level. Interventricular septum thickness and BSA were also found to be correlated with diameters of the sinus of Valsalva and the ascending and descending aorta. For males, there were no correlations between systolic or diastolic blood pressure and aortic diameter at the level of the sinus of Valsalva and ascending and descending aorta. For females, there was a significant correlation between systolic blood pressure and the diameter of the sinus of Valsalva (P = 0.008); all other relationships between blood pressure and aortic diameter were non-significant. Interaction between gender and IVS thickness, and between gender and age, was significant at the level of the sinus of Valsalva (P = 0.012 and P = 0.019, respectively).
Multiple regression analysis
| Response . | P-value . | ||
|---|---|---|---|
| . | Sinus of Valsalva . | Ascending aorta . | Descending aorta . |
| Gender | <0.0001 | 0.004 | 0.042 |
| BSA | 0.008 | 0.010 | 0.035 |
| IVS thickness | <0.0001 | 0.017 | 0.040 |
| Age | <0.001 | 0.082 | 0.246 |
| SBP | 0.693 | 0.515 | 0.549 |
| DBP | 0.562 | 0.796 | 0.489 |
| Creatinine | 0.461 | 0.818 | 0.312 |
| Gender: BSA interaction | 0.143 | 0.941 | 0.922 |
| Gender: IVS thickness interaction | 0.012 | 0.234 | 0.132 |
| Gender: age interaction | 0.019 | 0.184 | 0.804 |
| Gender: SBP interaction | 0.930 | 0.966 | 0.639 |
| Gender: DBP interaction | 0.497 | 0.468 | 0.766 |
| Gender: creatinine interaction | 0.628 | 0.603 | 0.677 |
| Response . | P-value . | ||
|---|---|---|---|
| . | Sinus of Valsalva . | Ascending aorta . | Descending aorta . |
| Gender | <0.0001 | 0.004 | 0.042 |
| BSA | 0.008 | 0.010 | 0.035 |
| IVS thickness | <0.0001 | 0.017 | 0.040 |
| Age | <0.001 | 0.082 | 0.246 |
| SBP | 0.693 | 0.515 | 0.549 |
| DBP | 0.562 | 0.796 | 0.489 |
| Creatinine | 0.461 | 0.818 | 0.312 |
| Gender: BSA interaction | 0.143 | 0.941 | 0.922 |
| Gender: IVS thickness interaction | 0.012 | 0.234 | 0.132 |
| Gender: age interaction | 0.019 | 0.184 | 0.804 |
| Gender: SBP interaction | 0.930 | 0.966 | 0.639 |
| Gender: DBP interaction | 0.497 | 0.468 | 0.766 |
| Gender: creatinine interaction | 0.628 | 0.603 | 0.677 |
Sinus of Valsalva: P-value is <0.05 for variables gender, body surface area (BSA), interventricular septum (IVS) thickness, and age. Ascending and descending aorta: P-value is <0.05 for variables gender, BSA, and IVS thickness. According to the above multiple regression analysis, the effect of these variables is statistically significant at the 0.05 alpha level. SBP, systolic blood pressure; DBP, diastolic blood pressure.
Multiple regression analysis
| Response . | P-value . | ||
|---|---|---|---|
| . | Sinus of Valsalva . | Ascending aorta . | Descending aorta . |
| Gender | <0.0001 | 0.004 | 0.042 |
| BSA | 0.008 | 0.010 | 0.035 |
| IVS thickness | <0.0001 | 0.017 | 0.040 |
| Age | <0.001 | 0.082 | 0.246 |
| SBP | 0.693 | 0.515 | 0.549 |
| DBP | 0.562 | 0.796 | 0.489 |
| Creatinine | 0.461 | 0.818 | 0.312 |
| Gender: BSA interaction | 0.143 | 0.941 | 0.922 |
| Gender: IVS thickness interaction | 0.012 | 0.234 | 0.132 |
| Gender: age interaction | 0.019 | 0.184 | 0.804 |
| Gender: SBP interaction | 0.930 | 0.966 | 0.639 |
| Gender: DBP interaction | 0.497 | 0.468 | 0.766 |
| Gender: creatinine interaction | 0.628 | 0.603 | 0.677 |
| Response . | P-value . | ||
|---|---|---|---|
| . | Sinus of Valsalva . | Ascending aorta . | Descending aorta . |
| Gender | <0.0001 | 0.004 | 0.042 |
| BSA | 0.008 | 0.010 | 0.035 |
| IVS thickness | <0.0001 | 0.017 | 0.040 |
| Age | <0.001 | 0.082 | 0.246 |
| SBP | 0.693 | 0.515 | 0.549 |
| DBP | 0.562 | 0.796 | 0.489 |
| Creatinine | 0.461 | 0.818 | 0.312 |
| Gender: BSA interaction | 0.143 | 0.941 | 0.922 |
| Gender: IVS thickness interaction | 0.012 | 0.234 | 0.132 |
| Gender: age interaction | 0.019 | 0.184 | 0.804 |
| Gender: SBP interaction | 0.930 | 0.966 | 0.639 |
| Gender: DBP interaction | 0.497 | 0.468 | 0.766 |
| Gender: creatinine interaction | 0.628 | 0.603 | 0.677 |
Sinus of Valsalva: P-value is <0.05 for variables gender, body surface area (BSA), interventricular septum (IVS) thickness, and age. Ascending and descending aorta: P-value is <0.05 for variables gender, BSA, and IVS thickness. According to the above multiple regression analysis, the effect of these variables is statistically significant at the 0.05 alpha level. SBP, systolic blood pressure; DBP, diastolic blood pressure.
Discussion
The present study provides more evidence for a high prevalence of ascending aorta dilation and aneurysms in male patients with FD compared with the normal population. Females with FD also developed dilation at the sinus of Valsalva and ascending aorta, but at a significantly lower rate than males, and ∼15–20 years later. Dilation appeared to be independent from cardiovascular risk factors.
The lower prevalence and delayed onset of aortic dilation in female patients is consistent with previous studies which have shown that compared with males, females are less prone to developed major structural arterial change.11,12 It is also in line with the body of evidence showing that disease severity is generally more variable and of later onset in females than in males, most probably as a result of the X-linked manner of inheritance of the condition.3–5,9 In male patients, dilation of the aorta at the level of the sinus of Valsalva and ascending aorta was observed in a comparable manner, in 32.7% and 29.6% of patients, respectively. Previous echocardiographic studies in smaller groups of patients have reported aortic root dilation in ∼30–56% of males, although the criteria used to determine dilation vary.9–12 The observed values in the present study, which examined unselected patients, likely represent a more realistic estimate of the prevalence of thoracic aortic dilation.
Asymptomatic thoracic fusiform aneurysms, involving two segments of the aorta as well as the aortic root and ascending aorta, were found in 9.6% of male patients.19 It is interesting to note that no patient had aortic dilation and/or aneurysm in the aortic arch or the descending aorta. Although the number of patients affected was too small to clearly delineate the risk of aneurysm, the results suggest that males are at relatively high risk of developing aortic aneurysms by the fifth decade of life.
The majority of patients with FD who had aortic dilation or aneurysm were young men, most of whom were either normotensive or had controlled hypertension and had one or more cardiovascular risk factors.8,20 Multivariate regression analysis revealed that gender, age, and IVS thickness were strongly associated with dilation at the sinus of Valsalva, but that dilation was not associated with systolic and/or diastolic blood pressure or other cardiovascular risk factors. In a previous study, we reported the absence of atherosclerotic plaques in the CCA in patients with FD relative to a control population among whom 34% were found to have atherosclerotic plaques.8 These results suggest that the conduit arteries may be protected from atherosclerosis in FD. Therefore, atherosclerosis and hypertension do not appear to play a significant role in the development of aortic dilation in patients with FD.
Aortic dilation can lead to aortic regurgitation, dissection, and/or rupture.21,22 However, to the best of our knowledge, aortic dissection or rupture has not been reported in FD. In patients with another genetic condition, Marfan syndrome, increased aortic stiffness often leads to early aortic dissection.23 In a recent study, Kalliokoski et al. reported that carotid, brachial, and aortic IMT were significantly increased in 17 Fabry patients compared with 34 healthy controls, but no differences were obtained in arterial compliance (stiffness) between the groups.24 The observation of an absence of functional aortic changes in Fabry patients, despite the presence of structural aortic anomalies, may explain why the risk of aortic dissection could be relatively limited in these patients.
Fabry disease has features (aortic dilation/aneurysm) in common with Marfan syndrome [fibrillin-1 (FBN1) gene] and other autosomal dominant disorders, such as Ehlers–Danlos syndrome (inborn defect of collagen type III, gene COL3A1) and familial aortic aneurusm/dissection.18,25 In these disorders, weakening of the aortic wall is mainly caused by cystic medial necrosis.26 This process is characterized by degeneration of elastic fibres and collagen in the medial layer of the aorta, and subsequent accumulation of mucoid material. Mutations of the FBN1 and COL3A1 genes may produce a spectrum of connective tissue disorders that lead to abnormal or reduced matrix deposition in the aorta and the subsequent development of an aneurysm.
The close correlation between thoracic aortic diameters and IVS thickness might suggest a common pathogenic mechanism. In a recent study, we detected a circulating growth-promoting factor in plasma from patients with FD that leads, in vitro, to both hypertrophy and hyperplasia of cardiomyocytes and vascular smooth muscle cells.20 We observed that the progressive development of LVH and increasing thickness of the CCA intima–media occurred concomitantly, and independently of blood pressure. The correlations between CCA IMT and IVS thickness and between IVS thickness and aortic diameter suggest that the same pathogenic mechanism contributes to the development of LVH, CCA intima–media thickening and thoracic aorta dilation/aneurysm. On the basis of these findings, IVS thickening may be a clinically relevant indicator of thoracic aortic dilation. Our hypothesis is supported by the work of Aerts et al.27 showing lyso-Gb3 as potential common growth-promoting factor.
The high prevalence of thoracic aortic dilation and aneurysm in FD, accompanied by normal blood pressure, indicates that FD should be treated as a cardiovascular disease in its own right. Longitudinal follow-up of a large cohort of patients is necessary to determine the natural history of aortic dilation and evaluate the risk of serious complications such as dissection and/or rupture. We suggest, however, that aortic diameter should be monitored in male patients with FD disease, particularly those who are aged ≥40 years or have severe LVH.
This study has certain limitations that should be acknowledged. We could not obtain all measurements using both echocardiographic and MRI depending on their limited availability in all centres involved in the study. It was not possible to obtain measurements of the aortic diameter at the three different levels in all patients. For defining normal and abnormal dimensions of the aorta, we used the international criteria and not an age-matched control group. In a future study, it would be of interest to examine the natural progression of dilation in greater detail.
In conclusion, the findings of this observational study, which we believe is the most comprehensive evaluation of thoracic aortic dilation in patients with FD conducted to date, suggest that the aortic remodelling process in FD primarily affects the aortic root and ascending aorta, mainly in male patients. Dilation appears at a younger age in patients with FD compared with the general population. As described previously, hypertension and atherosclerosis do not appear to be as important in the development of thoracic aortic dilation in FD as they are in the general population. Thus, FD should be included in the list of hereditary diseases that are associated with ascending thoracic dilation/aneurysm.
Acknowledgements
Editorial assistance to the authors was provided by Harriet Crofts (Oxford PharmaGenesis Ltd, Oxford, UK).
Conflict of interest: none declared.
References
Author notes
F.B. and S.D.Q. contributed equally to this work.
- aorta
- fabry disease
- echocardiography
- cardiovascular diseases
- heart disease risk factors
- dilatation of aorta
- descending aorta
- aneurysm
- ascending aorta
- descending thoracic aorta
- dilatation, pathologic
- ethics committees
- sinus of valsalva
- heart
- cardiac mri
- vasculature
- aortic diameter
- diameter
- clinical trial registration