-
PDF
- Split View
-
Views
-
Cite
Cite
Nobuyuki Murakoshi, DongZhu Xu, Toshimi Sairenchi, Miyako Igarashi, Fujiko Irie, Takuji Tomizawa, Hiroshi Tada, Yukio Sekiguchi, Kazumasa Yamagishi, Hiroyasu Iso, Iwao Yamaguchi, Hitoshi Ota, Kazutaka Aonuma, Prognostic impact of supraventricular premature complexes in community-based health checkups: The Ibaraki Prefectural Health Study, European Heart Journal, Volume 36, Issue 3, 14 January 2015, Pages 170–178, https://doi.org/10.1093/eurheartj/ehu407
Close - Share Icon Share
Abstract
The long-term prognosis of subjects with supraventricular premature complexes (SVPCs) remains unclear in the general population. The aim of this study was to examine the prognostic significance of SVPCs in community-based health checkups.
We assessed 63 197 individuals (mean age, 58.8 ± 9.9 years; 67.6% women) who participated in annual community-based health checkups in 1993 and were followed until 2008. The primary endpoint was stroke death, cardiovascular death (CVD), or all-cause death during a 14-year mean follow-up, and the secondary endpoint was first atrial fibrillation (AF) event in subjects without self-reported heart diseases or AF at baseline. Compared with subjects without SVPCs, the multivariate-adjusted hazard ratios (HRs) [95% confidence interval (CI)] of stroke death, CVD, and all-cause death in subjects with SVPCs were 1.24 (0.98–1.56) for men and 1.63 (1.30–2.05) for women, 1.22 (1.04–1.44) for men and 1.48 (1.25–1.74) for women, and 1.08 (0.99–1.18) for men and 1.21 (1.09–1.34) for women, respectively. Atrial fibrillation occurred in 386 subjects during the follow-up (1.05/1000 person-years). The presence of SVPCs at baseline was the significant predictor of AF onset [HRs (95% CI): 4.87 (3.61–6.57) for men and 3.87 (2.69–5.57) for women]. Propensity score matched analyses also revealed the presence of SVPCs was significantly associated with increased risks of AF incidence and CVD even after adjusting the potential confounders.
The presence of SVPCs in 12-lead electrocardiograms was a strong predictor of AF development, and associated with increased risk of CVD in general population.
See page 145 for the editorial comment on this article (doi:10.1093/eurheartj/ehu432)
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and has been further increasing with the advent of the ageing society.1,2 Advancing age, male sex, hypertension, obesity, and presence of organic heart disease are major risk factors for AF.3,4 Electrocardiograms (ECGs), the most widely used and inexpensive tool for screening of the cardiovascular status, were reported to be useful for risk stratification of AF. P wave prolongation and/or PR interval prolongation were shown to be predictive findings for AF.5,6 Moreover, left ventricular hypertrophy, ST segment and T wave (ST-T) abnormalities, and premature complexes are associated with an increased risk of AF onset.7 Recently, the importance of SVPCs in the initiation and maintenance of AF has been suggested in several studies. Excessive supraventricular premature complexes (SVPCs) documented by Holter monitoring were reported to be associated with the development of AF, stroke, and death.8 However, there is little information regarding the long-term prognosis of subjects with documented SVPCs in the general population. It also remains unclear whether SVPCs observed spontaneously during health checkups are really associated with an increased risk of AF development.
In this study, we analysed a large-scale database from the Ibaraki Prefectural Health Study (IPHS), a community-based cohort study conducted by the Ibaraki prefectural government from 1993 to 2008, to assess the electrocardiographic risk for long-term mortality and prognostic significance of SVPCs. We further assessed the risk of SVPCs for new onset of AF.
Methods
Study population
The Ibaraki prefectural government launched the IPHS community-based prospective cohort study in 1993. The detailed study protocol and selection procedures have been published previously.9–12 Briefly, 192 125 residents (63 379 men, 128 746 women) participated in health checkup conducted by the Ibaraki Health Service Association in 1993. The participation rate of the health checkups was 36.4% in the study area. Among them, 97 042 residents (33 130 men, 63 912 women) aged 40–79 years whose vital status had been followed-up were enrolled in the study. There were 279 subjects whose cause of death could not be identified, and they were excluded from the study. We next excluded 27 382 subjects who did not participate in the 1996 health checkup because they were not followed up for at least 1 year, and 1250 subjects who lacked complete data. We finally excluded subjects who had received medical treatment for heart disease (n = 4099) and subjects with AF at baseline (n = 835). Consequently, a total of 63 197 individuals (20 492 men, 42 705 women) were included in this study. Informed consent was obtained from community representatives to conduct an epidemiological study based on the guidelines of the Council for International Organizations of Medical Science. The Ethics Committee of Ibaraki Prefecture approved this study.
Baseline examinations
Standard resting 12-lead ECGs were recorded for ∼15 s at a paper speed of 25 mm/s. All ECGs were recorded by ECG-8300 (Nihon Kohden, Tokyo, Japan), and were diagnosed by educated cardiologists. Atrial fibrillation was diagnosed by ECG findings of irregular RR intervals and f waves, and SVPC was defined based on premature P-like wave followed by narrow QRS complexes (including blocked SVPC and aberrant conduction). The baseline ECG characteristics were based on a 3-year database from 1993 to 1995.
We also measured several potential confounders: smoking status; alcohol drinking habit; systolic blood pressure; diastolic blood pressure; body mass index (BMI); plasma glucose concentration; total cholesterol; high-density lipoprotein cholesterol; triglyceride; and estimated glomerular filtration rate (eGFR). The subjects completed a questionnaire regarding their health habits (smoking and drinking habits), known diseases, and medications, which was checked by specially trained nurses. The blood pressures were measured by trained observers using a standard mercury sphygmomanometer on the right arm of seated participants. The height and weight in light clothing were also measured, and the BMI was calculated as the weight (kg) divided by the square of the height (m). Diabetes was determined by a plasma glucose level of ≥126 mg/dL in the fasting state, plasma glucose level of ≥200 mg/dL in the normal daily state, or treatment of diabetes mellitus. Serum creatinine was measured by a modified method of Jaffe's reaction using an automated analyzer (RX-30; Nihon Denshi Inc., Tokyo, Japan). The eGFR was calculated using the abbreviated equation developed at the Cleveland Clinic Laboratory for the Modification of Diet in Renal Disease as follows: eGFR (mL/min/1.73 m2) = 194 × Cr−1.094 × Age−0.287 (×0.739 for women).
Endpoint determination and follow-up
The primary endpoint was death from total stroke (including ischaemic and haemorrhagic strokes), any cardiovascular cause, or all-cause, and the secondary endpoint was incidence of AF. Mortality surveillance was conducted by a systematic review of the death certificates and resident registration in cooperation with the public health centres and municipal government offices. The causes of death were coded according to the International Classification of Diseases (ICD), 9th revision and 10th revision. The cause-specific mortality was separately determined for stroke death (ICD-9 codes 430–438, ICD-10 codes I60–I69), cardiovascular death (CVD) (ICD-9 codes 393–459, ICD-10 codes I00–I99), and all-cause death (ICD-9 codes 001-999, ICD-10 codes A00–Y89). The follow-up was conducted until the end of 2008, and the mean follow-up period was 14.3 years in the primary analysis. Persons who moved out of the communities or died during the follow-up were censored at the date of moving out or death. In the secondary analysis, the first 3 years (from 1993 to 1995) were excluded from the follow-up periods. If a subject could not receive health checkup of a certain year, he was considered as a censored case at the time. The new-onset AF was defined by ECGs recorded in annual community-based health checkups every year. When AF was first detected by the annual ECGs during the follow-up period, we defined the subject as a case of AF incidence. The mean follow-up period became 5.8 years. We did not include atrial flutter into clinical endpoint (AF onset) because it differed in the pathogenesis from AF.
Statistical analysis
Continuous variables are presented as means ± SD, and categorical variables are presented as percentages in each group. Differences between two groups (with SVPCs vs. without SVPCs) were tested by an unpaired t-test for continuous variables and the Chi-square test for categorical variables. The sex-specific event-free ratios for stroke, CVD, all-cause death, and AF incidence were estimated by the Kaplan–Meier method, and compared using the log-rank test. The sex-specific hazard ratio (HR) and 95% confidence interval (CI) for each endpoint were calculated and multivariate-adjusted survival curves were plotted using multivariate Cox proportional hazard models. The following models were generated to determine the influence of potential confounders on the relationships between SVPCs and endpoints: (1) unadjusted; (2) adjusted for age; (3) adjusted for the following potential confounders: age, BMI, systolic blood pressure, treatment for hypertension, past history of stroke, diabetic status, total cholesterol, HDL-cholesterol, triglyceride, eGFR, smoking status, alcohol habit, SVPCs, VPCs, sinus bradycardia, sinus tachycardia, atrioventricular conduction block, left atrial overload, right bundle branch block, left bundle branch block, voltage criteria for LVH, ST-T abnormalities, and abnormal Q waves. The effects of SVPC burden (single or multiple SVPCs) were analysed using dummy variables. To investigate interaction between SVPCs and sex in each outcome, SVPCs, sex, and interaction term (SVPCs × sex) were also included in the multivariate analysis. We further performed propensity score-matched analysis to adjust the differences in baseline characteristics between the subjects with and without SVPCs. We matched the subjects on the potential confounders described above. All reported P-values were two-sided, and values of P < 0.05 were considered to indicate statistical significance. All statistical analyses were performed with SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
The baseline characteristics of the 63 197 participants are shown in Table 1. The mean age was 58.8 ± 9.9 years, and 67.6% of the subjects were women. Supraventricular premature complexes were documented in 3858 subjects (6.10%). The subjects with SVPCs were significantly older, and more likely to have hypertension, and lower BMI, total cholesterol, triglyceride, and eGFR than those without SVPCs. The percentages of current smokers and current drinkers were higher in the subjects with SVPCs than in those without SVPCs.
Baseline characteristics
| . | All . | With SVPCs . | Without SVPCs . | P-value . |
|---|---|---|---|---|
| n | 63 197 | 3858 (6.10) | 59 339 (93.9) | |
| Sex (%, women) | 42 705 (67.6) | 2 208 (57.2) | 40 497 (68.2) | <0.001 |
| Age (year-old) | 58.8 ± 9.9 | 64.8 ± 8.5 | 58.4 ± 9.9 | <0.001 |
| Past history of stroke | 535 (0.85) | 62 (1.6) | 473 (0.80) | <0.001 |
| Height | 153.2 ± 8.0 | 152.8 ± 8.3 | 153.2 ± 8.0 | <0.001 |
| Weight | 55.2 ± 9.0 | 53.6 ± 9.1 | 55.3 ± 9.0 | <0.001 |
| Body mass index (kg/m2) | 23.5 ± 3.1 | 22.9 ± 3.2 | 23.5 ± 3.1 | <0.001 |
| Systolic blood pressure (mmHg) | 133.1 ± 17.6 | 137.5 ± 17.7 | 132.9 ± 17.6 | <0.001 |
| Diastolic blood pressure (mmHg) | 78.7 ± 10.6 | 79.4 ± 10.6 | 78.6 ± 10.6 | <0.001 |
| Anti-hypertensive therapy (%) | 12 242 (19.4) | 987 (25.6) | 11 255 (19.0) | <0.001 |
| Diabetes mellitus (%) | 3002 (4.8) | 176 (4.6) | 2826 (4.8) | 0.5705 |
| Total cholesterol (mg/dL) | 203.0 ± 34.8 | 198.6 ± 34.4 | 203.2 ± 34.8 | <0.001 |
| HDL-cholesterol (mg/dL) | 55.4 ± 14.3 | 56.5 ± 14.7 | 55.3 ± 14.3 | <0.001 |
| Triglyceride (mg/dL) | 139.1 ± 83.5 | 128.8 ± 73.5 | 139.7 ± 84.0 | <0.001 |
| eGFR (mL/min) | 90.9 ± 22.2 | 86.4 ± 21.5 | 91.2 ± 22.2 | <0.001 |
| Current smoking (%) | 17 766 (28.1) | 1364 (35.4) | 16 402 (27.6) | <0.001 |
| Current alcohol (%) | 11 870 (18.8) | 892 (23.2) | 10 978 (18.7) | <0.001 |
| Electrocardiographic findings | ||||
| Ventricular premature complexes (%) | 3437 (5.46) | 442 (11.50) | 2995 (5.05) | <0.001 |
| Sinus bradycardia (%) | 624 (0.99) | 71 (1.84) | 553 (0.93) | <0.001 |
| Sinus tachycardia (%) | 977 (1.54) | 49 (1.27) | 928 (1.56) | 0.152 |
| Atrioventricular block (%) | 627 (1.00) | 72 (1.87) | 555 (0.94) | <0.001 |
| Left atrial overload (%) | 104 (0.16) | 6 (0.16) | 98 (0.17) | 0.886 |
| Right bundle branch block (%) | 2379 (3.78) | 228 (5.91) | 2151 (3.62) | <0.001 |
| Left bundle branch block (%) | 125 (0.20) | 9 (0.23) | 116 (0.20) | 0.644 |
| Voltage criteria for LVH (%) | 3132 (4.96) | 296 (7.67) | 2835 (4.78) | <0.001 |
| ST-T abnormalities (%) | 3713 (5.89) | 293 (7.59) | 3420 (5.76) | <0.001 |
| Abnormal Q wave (%) | 433 (0.69) | 46 (1.19) | 387 (0.65) | <0.001 |
| . | All . | With SVPCs . | Without SVPCs . | P-value . |
|---|---|---|---|---|
| n | 63 197 | 3858 (6.10) | 59 339 (93.9) | |
| Sex (%, women) | 42 705 (67.6) | 2 208 (57.2) | 40 497 (68.2) | <0.001 |
| Age (year-old) | 58.8 ± 9.9 | 64.8 ± 8.5 | 58.4 ± 9.9 | <0.001 |
| Past history of stroke | 535 (0.85) | 62 (1.6) | 473 (0.80) | <0.001 |
| Height | 153.2 ± 8.0 | 152.8 ± 8.3 | 153.2 ± 8.0 | <0.001 |
| Weight | 55.2 ± 9.0 | 53.6 ± 9.1 | 55.3 ± 9.0 | <0.001 |
| Body mass index (kg/m2) | 23.5 ± 3.1 | 22.9 ± 3.2 | 23.5 ± 3.1 | <0.001 |
| Systolic blood pressure (mmHg) | 133.1 ± 17.6 | 137.5 ± 17.7 | 132.9 ± 17.6 | <0.001 |
| Diastolic blood pressure (mmHg) | 78.7 ± 10.6 | 79.4 ± 10.6 | 78.6 ± 10.6 | <0.001 |
| Anti-hypertensive therapy (%) | 12 242 (19.4) | 987 (25.6) | 11 255 (19.0) | <0.001 |
| Diabetes mellitus (%) | 3002 (4.8) | 176 (4.6) | 2826 (4.8) | 0.5705 |
| Total cholesterol (mg/dL) | 203.0 ± 34.8 | 198.6 ± 34.4 | 203.2 ± 34.8 | <0.001 |
| HDL-cholesterol (mg/dL) | 55.4 ± 14.3 | 56.5 ± 14.7 | 55.3 ± 14.3 | <0.001 |
| Triglyceride (mg/dL) | 139.1 ± 83.5 | 128.8 ± 73.5 | 139.7 ± 84.0 | <0.001 |
| eGFR (mL/min) | 90.9 ± 22.2 | 86.4 ± 21.5 | 91.2 ± 22.2 | <0.001 |
| Current smoking (%) | 17 766 (28.1) | 1364 (35.4) | 16 402 (27.6) | <0.001 |
| Current alcohol (%) | 11 870 (18.8) | 892 (23.2) | 10 978 (18.7) | <0.001 |
| Electrocardiographic findings | ||||
| Ventricular premature complexes (%) | 3437 (5.46) | 442 (11.50) | 2995 (5.05) | <0.001 |
| Sinus bradycardia (%) | 624 (0.99) | 71 (1.84) | 553 (0.93) | <0.001 |
| Sinus tachycardia (%) | 977 (1.54) | 49 (1.27) | 928 (1.56) | 0.152 |
| Atrioventricular block (%) | 627 (1.00) | 72 (1.87) | 555 (0.94) | <0.001 |
| Left atrial overload (%) | 104 (0.16) | 6 (0.16) | 98 (0.17) | 0.886 |
| Right bundle branch block (%) | 2379 (3.78) | 228 (5.91) | 2151 (3.62) | <0.001 |
| Left bundle branch block (%) | 125 (0.20) | 9 (0.23) | 116 (0.20) | 0.644 |
| Voltage criteria for LVH (%) | 3132 (4.96) | 296 (7.67) | 2835 (4.78) | <0.001 |
| ST-T abnormalities (%) | 3713 (5.89) | 293 (7.59) | 3420 (5.76) | <0.001 |
| Abnormal Q wave (%) | 433 (0.69) | 46 (1.19) | 387 (0.65) | <0.001 |
The characteristics are expressed as mean ± SD or number of subjects (%). The electrocardiographic characteristics were based on a 3-year database from 1993 to 1995.
HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; LVH, left ventricular hypertrophy; ST-T, ST-segment and T-wave.
Baseline characteristics
| . | All . | With SVPCs . | Without SVPCs . | P-value . |
|---|---|---|---|---|
| n | 63 197 | 3858 (6.10) | 59 339 (93.9) | |
| Sex (%, women) | 42 705 (67.6) | 2 208 (57.2) | 40 497 (68.2) | <0.001 |
| Age (year-old) | 58.8 ± 9.9 | 64.8 ± 8.5 | 58.4 ± 9.9 | <0.001 |
| Past history of stroke | 535 (0.85) | 62 (1.6) | 473 (0.80) | <0.001 |
| Height | 153.2 ± 8.0 | 152.8 ± 8.3 | 153.2 ± 8.0 | <0.001 |
| Weight | 55.2 ± 9.0 | 53.6 ± 9.1 | 55.3 ± 9.0 | <0.001 |
| Body mass index (kg/m2) | 23.5 ± 3.1 | 22.9 ± 3.2 | 23.5 ± 3.1 | <0.001 |
| Systolic blood pressure (mmHg) | 133.1 ± 17.6 | 137.5 ± 17.7 | 132.9 ± 17.6 | <0.001 |
| Diastolic blood pressure (mmHg) | 78.7 ± 10.6 | 79.4 ± 10.6 | 78.6 ± 10.6 | <0.001 |
| Anti-hypertensive therapy (%) | 12 242 (19.4) | 987 (25.6) | 11 255 (19.0) | <0.001 |
| Diabetes mellitus (%) | 3002 (4.8) | 176 (4.6) | 2826 (4.8) | 0.5705 |
| Total cholesterol (mg/dL) | 203.0 ± 34.8 | 198.6 ± 34.4 | 203.2 ± 34.8 | <0.001 |
| HDL-cholesterol (mg/dL) | 55.4 ± 14.3 | 56.5 ± 14.7 | 55.3 ± 14.3 | <0.001 |
| Triglyceride (mg/dL) | 139.1 ± 83.5 | 128.8 ± 73.5 | 139.7 ± 84.0 | <0.001 |
| eGFR (mL/min) | 90.9 ± 22.2 | 86.4 ± 21.5 | 91.2 ± 22.2 | <0.001 |
| Current smoking (%) | 17 766 (28.1) | 1364 (35.4) | 16 402 (27.6) | <0.001 |
| Current alcohol (%) | 11 870 (18.8) | 892 (23.2) | 10 978 (18.7) | <0.001 |
| Electrocardiographic findings | ||||
| Ventricular premature complexes (%) | 3437 (5.46) | 442 (11.50) | 2995 (5.05) | <0.001 |
| Sinus bradycardia (%) | 624 (0.99) | 71 (1.84) | 553 (0.93) | <0.001 |
| Sinus tachycardia (%) | 977 (1.54) | 49 (1.27) | 928 (1.56) | 0.152 |
| Atrioventricular block (%) | 627 (1.00) | 72 (1.87) | 555 (0.94) | <0.001 |
| Left atrial overload (%) | 104 (0.16) | 6 (0.16) | 98 (0.17) | 0.886 |
| Right bundle branch block (%) | 2379 (3.78) | 228 (5.91) | 2151 (3.62) | <0.001 |
| Left bundle branch block (%) | 125 (0.20) | 9 (0.23) | 116 (0.20) | 0.644 |
| Voltage criteria for LVH (%) | 3132 (4.96) | 296 (7.67) | 2835 (4.78) | <0.001 |
| ST-T abnormalities (%) | 3713 (5.89) | 293 (7.59) | 3420 (5.76) | <0.001 |
| Abnormal Q wave (%) | 433 (0.69) | 46 (1.19) | 387 (0.65) | <0.001 |
| . | All . | With SVPCs . | Without SVPCs . | P-value . |
|---|---|---|---|---|
| n | 63 197 | 3858 (6.10) | 59 339 (93.9) | |
| Sex (%, women) | 42 705 (67.6) | 2 208 (57.2) | 40 497 (68.2) | <0.001 |
| Age (year-old) | 58.8 ± 9.9 | 64.8 ± 8.5 | 58.4 ± 9.9 | <0.001 |
| Past history of stroke | 535 (0.85) | 62 (1.6) | 473 (0.80) | <0.001 |
| Height | 153.2 ± 8.0 | 152.8 ± 8.3 | 153.2 ± 8.0 | <0.001 |
| Weight | 55.2 ± 9.0 | 53.6 ± 9.1 | 55.3 ± 9.0 | <0.001 |
| Body mass index (kg/m2) | 23.5 ± 3.1 | 22.9 ± 3.2 | 23.5 ± 3.1 | <0.001 |
| Systolic blood pressure (mmHg) | 133.1 ± 17.6 | 137.5 ± 17.7 | 132.9 ± 17.6 | <0.001 |
| Diastolic blood pressure (mmHg) | 78.7 ± 10.6 | 79.4 ± 10.6 | 78.6 ± 10.6 | <0.001 |
| Anti-hypertensive therapy (%) | 12 242 (19.4) | 987 (25.6) | 11 255 (19.0) | <0.001 |
| Diabetes mellitus (%) | 3002 (4.8) | 176 (4.6) | 2826 (4.8) | 0.5705 |
| Total cholesterol (mg/dL) | 203.0 ± 34.8 | 198.6 ± 34.4 | 203.2 ± 34.8 | <0.001 |
| HDL-cholesterol (mg/dL) | 55.4 ± 14.3 | 56.5 ± 14.7 | 55.3 ± 14.3 | <0.001 |
| Triglyceride (mg/dL) | 139.1 ± 83.5 | 128.8 ± 73.5 | 139.7 ± 84.0 | <0.001 |
| eGFR (mL/min) | 90.9 ± 22.2 | 86.4 ± 21.5 | 91.2 ± 22.2 | <0.001 |
| Current smoking (%) | 17 766 (28.1) | 1364 (35.4) | 16 402 (27.6) | <0.001 |
| Current alcohol (%) | 11 870 (18.8) | 892 (23.2) | 10 978 (18.7) | <0.001 |
| Electrocardiographic findings | ||||
| Ventricular premature complexes (%) | 3437 (5.46) | 442 (11.50) | 2995 (5.05) | <0.001 |
| Sinus bradycardia (%) | 624 (0.99) | 71 (1.84) | 553 (0.93) | <0.001 |
| Sinus tachycardia (%) | 977 (1.54) | 49 (1.27) | 928 (1.56) | 0.152 |
| Atrioventricular block (%) | 627 (1.00) | 72 (1.87) | 555 (0.94) | <0.001 |
| Left atrial overload (%) | 104 (0.16) | 6 (0.16) | 98 (0.17) | 0.886 |
| Right bundle branch block (%) | 2379 (3.78) | 228 (5.91) | 2151 (3.62) | <0.001 |
| Left bundle branch block (%) | 125 (0.20) | 9 (0.23) | 116 (0.20) | 0.644 |
| Voltage criteria for LVH (%) | 3132 (4.96) | 296 (7.67) | 2835 (4.78) | <0.001 |
| ST-T abnormalities (%) | 3713 (5.89) | 293 (7.59) | 3420 (5.76) | <0.001 |
| Abnormal Q wave (%) | 433 (0.69) | 46 (1.19) | 387 (0.65) | <0.001 |
The characteristics are expressed as mean ± SD or number of subjects (%). The electrocardiographic characteristics were based on a 3-year database from 1993 to 1995.
HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; LVH, left ventricular hypertrophy; ST-T, ST-segment and T-wave.
The ECG characteristics for the 3-year baseline are also shown in Table 1. In both men and women, VPCs, sinus bradycardia, atrioventricular block, right bundle branch block, LVH, ST-T abnormalities, and abnormal Q waves were more frequently observed in the subjects with SVPCs than in those without SVPCs, and the differences were statistically significant.
Primary analysis: long-term mortality in subjects with and without supraventricular premature complexes
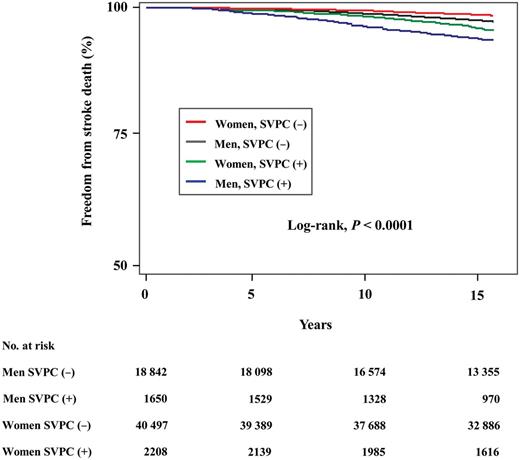
During the mean follow-up of 14.3 years, 1208 (563 men, 645 women) participants died from stroke in the cohort. Figure 1 shows the Kaplan–Meier curves of freedom from stroke death for the subjects with and without SVPCs. Both men and women with SVPCs were significantly more susceptible to death from stroke than those without SVPCs (the log-rank test, all P < 0.0001). At the 10-year follow-up, 3.9% of men with SVPCs had died from stroke compared with 1.4% of men without SVPCs, and 1.9% of women with SVPCs had died from stroke compared with 0.6% of women without SVPCs. Table 2 shows sex-specific unadjusted, age-adjusted, and multivariate-adjusted HRs and 95% confidence intervals (95% CIs) of SVPCs for stroke death. After adjustment for all the potential confounders, SVPCs were associated with the increased risk of death from stroke (Table 2). Although women with SVPCs tended to have more susceptible to stroke death than men with SVPCs, the difference was not statistically significant.
Hazard ratios of supraventricular complexes for stroke death, cardiovascular death, all-cause death, or atrial fibrillation onset
| . | Model 1: unadjusted . | Model 2: age-adjusted . | Model 3: multivariate . | P for interaction . | |||
|---|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | |
| Stroke death | |||||||
| Men | 2.317 | 1.846–2.909 | 1.314 | 1.044–1.655 | 1.237 | 0.980–1.561 | 0.078 |
| Women | 3.095 | 2.477–3.868 | 1.742 | 1.392–2.180 | 1.634 | 1.303–2.049 | |
| Cardiovascular death | |||||||
| Men | 2.175 | 1.855–2.550 | 1.272 | 1.083–1.494 | 1.220 | 1.037–1.436 | 0.027 |
| Women | 2.769 | 2.354–3.257 | 1.536 | 1.305–1.809 | 1.478 | 1.253–1.742 | |
| All-cause death | |||||||
| Men | 1.816 | 1.665–1.980 | 1.126 | 1.031–1.229 | 1.079 | 0.988–1.179 | 0.038 |
| Women | 2.070 | 1.867–2.295 | 1.252 | 1.128–1.389 | 1.205 | 1.085–1.338 | |
| Atrial fibrillation onset | |||||||
| Men | 6.662 | 5.012–8.855 | 5.336 | 3.984–7.147 | 4.871 | 3.613–6.566 | 0.631 |
| Women | 6.005 | 4.217–8.551 | 4.058 | 2.830–5.817 | 3.871 | 2.688–5.573 | |
| . | Model 1: unadjusted . | Model 2: age-adjusted . | Model 3: multivariate . | P for interaction . | |||
|---|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | |
| Stroke death | |||||||
| Men | 2.317 | 1.846–2.909 | 1.314 | 1.044–1.655 | 1.237 | 0.980–1.561 | 0.078 |
| Women | 3.095 | 2.477–3.868 | 1.742 | 1.392–2.180 | 1.634 | 1.303–2.049 | |
| Cardiovascular death | |||||||
| Men | 2.175 | 1.855–2.550 | 1.272 | 1.083–1.494 | 1.220 | 1.037–1.436 | 0.027 |
| Women | 2.769 | 2.354–3.257 | 1.536 | 1.305–1.809 | 1.478 | 1.253–1.742 | |
| All-cause death | |||||||
| Men | 1.816 | 1.665–1.980 | 1.126 | 1.031–1.229 | 1.079 | 0.988–1.179 | 0.038 |
| Women | 2.070 | 1.867–2.295 | 1.252 | 1.128–1.389 | 1.205 | 1.085–1.338 | |
| Atrial fibrillation onset | |||||||
| Men | 6.662 | 5.012–8.855 | 5.336 | 3.984–7.147 | 4.871 | 3.613–6.566 | 0.631 |
| Women | 6.005 | 4.217–8.551 | 4.058 | 2.830–5.817 | 3.871 | 2.688–5.573 | |
The variables included in the multivariate analyses were age, body mass index, systolic blood pressure, presence or absence of anti-hypertensive therapy, presence or absence of past history of stroke, presence or absence of diabetes, total cholesterol, high density lipoprotein cholesterol, triglyceride, estimated glomerular filtration rate, current smoking, drinking habit, and presence or absence of the following electrocardiographic findings: ventricular premature complexes, sinus bradycardia, sinus tachycardia, atrioventricular block, left atrial overload, right bundle branch block, left bundle branch block, voltage criteria for left ventricular hypertrophy, ST segment and T wave abnormalities, and abnormal Q waves.
HR, hazard ratio; CI, confidence interval.
Hazard ratios of supraventricular complexes for stroke death, cardiovascular death, all-cause death, or atrial fibrillation onset
| . | Model 1: unadjusted . | Model 2: age-adjusted . | Model 3: multivariate . | P for interaction . | |||
|---|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | |
| Stroke death | |||||||
| Men | 2.317 | 1.846–2.909 | 1.314 | 1.044–1.655 | 1.237 | 0.980–1.561 | 0.078 |
| Women | 3.095 | 2.477–3.868 | 1.742 | 1.392–2.180 | 1.634 | 1.303–2.049 | |
| Cardiovascular death | |||||||
| Men | 2.175 | 1.855–2.550 | 1.272 | 1.083–1.494 | 1.220 | 1.037–1.436 | 0.027 |
| Women | 2.769 | 2.354–3.257 | 1.536 | 1.305–1.809 | 1.478 | 1.253–1.742 | |
| All-cause death | |||||||
| Men | 1.816 | 1.665–1.980 | 1.126 | 1.031–1.229 | 1.079 | 0.988–1.179 | 0.038 |
| Women | 2.070 | 1.867–2.295 | 1.252 | 1.128–1.389 | 1.205 | 1.085–1.338 | |
| Atrial fibrillation onset | |||||||
| Men | 6.662 | 5.012–8.855 | 5.336 | 3.984–7.147 | 4.871 | 3.613–6.566 | 0.631 |
| Women | 6.005 | 4.217–8.551 | 4.058 | 2.830–5.817 | 3.871 | 2.688–5.573 | |
| . | Model 1: unadjusted . | Model 2: age-adjusted . | Model 3: multivariate . | P for interaction . | |||
|---|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . | HR . | 95% CI . | |
| Stroke death | |||||||
| Men | 2.317 | 1.846–2.909 | 1.314 | 1.044–1.655 | 1.237 | 0.980–1.561 | 0.078 |
| Women | 3.095 | 2.477–3.868 | 1.742 | 1.392–2.180 | 1.634 | 1.303–2.049 | |
| Cardiovascular death | |||||||
| Men | 2.175 | 1.855–2.550 | 1.272 | 1.083–1.494 | 1.220 | 1.037–1.436 | 0.027 |
| Women | 2.769 | 2.354–3.257 | 1.536 | 1.305–1.809 | 1.478 | 1.253–1.742 | |
| All-cause death | |||||||
| Men | 1.816 | 1.665–1.980 | 1.126 | 1.031–1.229 | 1.079 | 0.988–1.179 | 0.038 |
| Women | 2.070 | 1.867–2.295 | 1.252 | 1.128–1.389 | 1.205 | 1.085–1.338 | |
| Atrial fibrillation onset | |||||||
| Men | 6.662 | 5.012–8.855 | 5.336 | 3.984–7.147 | 4.871 | 3.613–6.566 | 0.631 |
| Women | 6.005 | 4.217–8.551 | 4.058 | 2.830–5.817 | 3.871 | 2.688–5.573 | |
The variables included in the multivariate analyses were age, body mass index, systolic blood pressure, presence or absence of anti-hypertensive therapy, presence or absence of past history of stroke, presence or absence of diabetes, total cholesterol, high density lipoprotein cholesterol, triglyceride, estimated glomerular filtration rate, current smoking, drinking habit, and presence or absence of the following electrocardiographic findings: ventricular premature complexes, sinus bradycardia, sinus tachycardia, atrioventricular block, left atrial overload, right bundle branch block, left bundle branch block, voltage criteria for left ventricular hypertrophy, ST segment and T wave abnormalities, and abnormal Q waves.
HR, hazard ratio; CI, confidence interval.
Kaplan–Meier curves of the association of supraventricular premature complexes with freedom from stroke death in the community-based general population. Both men and women with supraventricular premature complexes had a significantly higher risk of death from stroke than those without supraventricular premature complexes (the log-rank test, all P < 0.0001).
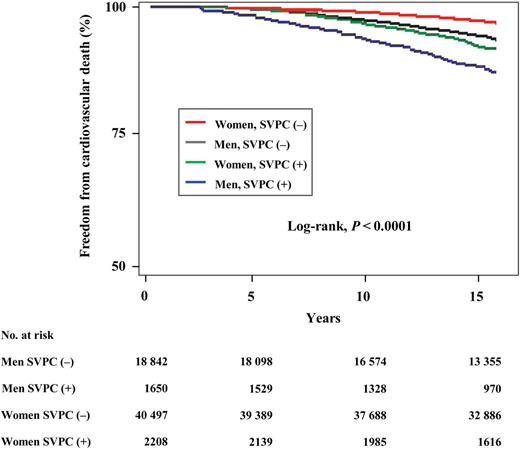
There were 2527 (1205 men, 1322 women) deaths from any cardiovascular cause. Similar to the curves of freedom from stroke death, both men and women with SVPCs had significantly higher mortality from cardiovascular causes than those without SVPCs (the log-rank test, all P < 0.0001; Figure 2). At the 10-year follow-up, 6.7% of men with SVPCs had died from cardiovascular causes compared with 2.8% of men without SVPCs, and 3.6% of women with SVPCs had died from cardiovascular causes compared with 1.2% of women without SVPCs. After adjustment for all the potential confounders, SVPCs were also associated with increased risk of CVD (Table 2). The impact of the presence or absence of SVPCs on cardiovascular mortality was significantly stronger in women than in men.
Kaplan–Meier curves of the association of supraventricular premature complexes with freedom from cardiovascular death in the community-based general population. Both men and women with supraventricular premature complexes had a significantly higher risk of death from cardiovascular causes than those without supraventricular premature complexes (the log-rank test, all P < 0.0001).
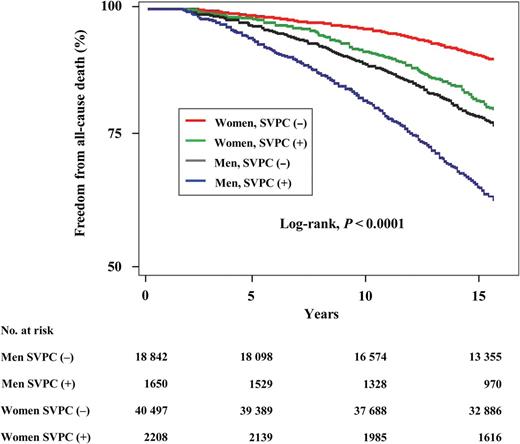
There were 8712 (4617 men, 4095 women) all-cause deaths. Both men and women with SVPCs had significantly higher mortality from all causes than those without SVPCs (the log-rank test, all P < 0.0001; Figure 3). At the 10-year follow-up, 18.2% of men with SVPCs had died compared with 10.8% of men without SVPCs, and 8.3% of women with SVPCs had died compared with 4.1% of women without SVPCs. After adjustment for all the potential confounders, the HRs of all-cause death for women and total subjects with SVPCs remained significant (Table 2). The impact of the presence or absence of SVPCs on all-cause death was also stronger in women than in men.
Kaplan–Meier curves of the association of supraventricular premature complexes with freedom from all-cause death in the community-based general population. Both men and women with supraventricular premature complexes had a significantly higher risk of all-cause death than those without supraventricular premature complexes (the log-rank test, all P < 0.0001).
Secondary analysis: atrial fibrillation incidence rates in subjects with and without supraventricular premature complexes
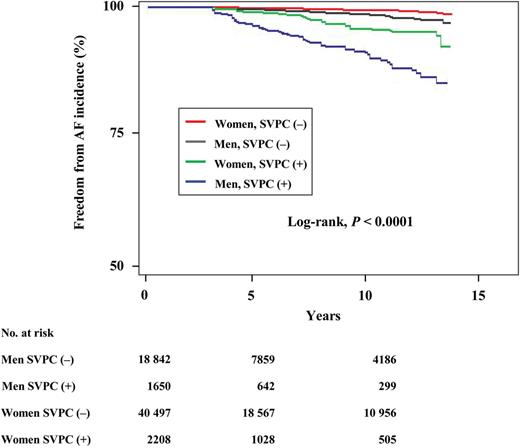
During the mean follow-up of 5.8 years, 386 (212 men, 174 women) participants developed AF (1.90, 0.69, and 1.05 per 1000 person-years for men, women, and total, respectively). Among 3858 subjects with SVPCs at baseline, 112 developed AF, while among subjects without SVPCs at baseline, 274 developed AF. The Kaplan–Meier curves of freedom from AF incidence clearly showed a separation between the subjects with and without SVPCs (the log-rank test, all P < 0.0001; Figure 4) for both men and women. At the 10-year follow-up, 9.9% of men with SVPCs had developed AF compared with 1.5% of men without SVPCs, and 4.2% of women with SVPCs had developed AF compared with 0.6% of women without SVPCs. Multivariate analyses revealed that the subjects with SVPCs had a 4.5-fold higher risk of AF incidence than those without SVPCs (Table 2). After adjustment for all the potential confounders, the presence of SVPCs remained significant for AF development. The presence of SVPCs in the baseline ECG was the strong predictor of AF incidence among the other ECG findings (Table 3).
Electrocardiographic risk for atrial fibrillation onset
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . |
| SVPCs | 4.871 | 3.613–6.566 | 3.871 | 2.688–5.573 |
| VPCs | 1.793 | 1.220–2.636 | 1.524 | 0.908–2.555 |
| Sinus bradycardia | 1.490 | 0.728–3.049 | 2.878 | 1.169–7.086 |
| Sinus tachycardia | 0.896 | 0.220–3.645 | 1.110 | 0.353–3.493 |
| Atrioventricular block | 0.800 | 0.296–2.160 | 3.852 | 1.575–9.424 |
| Left atrial overload | 0.864 | 0.117–6.370 | 0.000 | 0.000–0.000 |
| Right bundle branch block | 0.790 | 0.428–1.460 | 0.648 | 0.240–1.751 |
| Left bundle branch block | 3.342 | 0.820–13.624 | 0.000 | 0.000–0.000 |
| Voltage criteria for LVH | 1.338 | 0.883–20329 | 1.719 | 0.971–3.042 |
| ST-T abnormalities | 1.638 | 1.028–2.610 | 1.522 | 0.965–2.401 |
| Abnormal Q wave | 1.131 | 0.357–3.587 | 1.840 | 0.578–5.858 |
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . |
| SVPCs | 4.871 | 3.613–6.566 | 3.871 | 2.688–5.573 |
| VPCs | 1.793 | 1.220–2.636 | 1.524 | 0.908–2.555 |
| Sinus bradycardia | 1.490 | 0.728–3.049 | 2.878 | 1.169–7.086 |
| Sinus tachycardia | 0.896 | 0.220–3.645 | 1.110 | 0.353–3.493 |
| Atrioventricular block | 0.800 | 0.296–2.160 | 3.852 | 1.575–9.424 |
| Left atrial overload | 0.864 | 0.117–6.370 | 0.000 | 0.000–0.000 |
| Right bundle branch block | 0.790 | 0.428–1.460 | 0.648 | 0.240–1.751 |
| Left bundle branch block | 3.342 | 0.820–13.624 | 0.000 | 0.000–0.000 |
| Voltage criteria for LVH | 1.338 | 0.883–20329 | 1.719 | 0.971–3.042 |
| ST-T abnormalities | 1.638 | 1.028–2.610 | 1.522 | 0.965–2.401 |
| Abnormal Q wave | 1.131 | 0.357–3.587 | 1.840 | 0.578–5.858 |
HR, hazard ratio; CI, confidence interval; SVPCs, supraventricular premature complexes; VPCs, ventricular premature complexes; LVH, left ventricular hypertrophy; ST-T, ST-segment and T-wave.
Electrocardiographic risk for atrial fibrillation onset
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . |
| SVPCs | 4.871 | 3.613–6.566 | 3.871 | 2.688–5.573 |
| VPCs | 1.793 | 1.220–2.636 | 1.524 | 0.908–2.555 |
| Sinus bradycardia | 1.490 | 0.728–3.049 | 2.878 | 1.169–7.086 |
| Sinus tachycardia | 0.896 | 0.220–3.645 | 1.110 | 0.353–3.493 |
| Atrioventricular block | 0.800 | 0.296–2.160 | 3.852 | 1.575–9.424 |
| Left atrial overload | 0.864 | 0.117–6.370 | 0.000 | 0.000–0.000 |
| Right bundle branch block | 0.790 | 0.428–1.460 | 0.648 | 0.240–1.751 |
| Left bundle branch block | 3.342 | 0.820–13.624 | 0.000 | 0.000–0.000 |
| Voltage criteria for LVH | 1.338 | 0.883–20329 | 1.719 | 0.971–3.042 |
| ST-T abnormalities | 1.638 | 1.028–2.610 | 1.522 | 0.965–2.401 |
| Abnormal Q wave | 1.131 | 0.357–3.587 | 1.840 | 0.578–5.858 |
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | HR . | 95% CI . | HR . | 95% CI . |
| SVPCs | 4.871 | 3.613–6.566 | 3.871 | 2.688–5.573 |
| VPCs | 1.793 | 1.220–2.636 | 1.524 | 0.908–2.555 |
| Sinus bradycardia | 1.490 | 0.728–3.049 | 2.878 | 1.169–7.086 |
| Sinus tachycardia | 0.896 | 0.220–3.645 | 1.110 | 0.353–3.493 |
| Atrioventricular block | 0.800 | 0.296–2.160 | 3.852 | 1.575–9.424 |
| Left atrial overload | 0.864 | 0.117–6.370 | 0.000 | 0.000–0.000 |
| Right bundle branch block | 0.790 | 0.428–1.460 | 0.648 | 0.240–1.751 |
| Left bundle branch block | 3.342 | 0.820–13.624 | 0.000 | 0.000–0.000 |
| Voltage criteria for LVH | 1.338 | 0.883–20329 | 1.719 | 0.971–3.042 |
| ST-T abnormalities | 1.638 | 1.028–2.610 | 1.522 | 0.965–2.401 |
| Abnormal Q wave | 1.131 | 0.357–3.587 | 1.840 | 0.578–5.858 |
HR, hazard ratio; CI, confidence interval; SVPCs, supraventricular premature complexes; VPCs, ventricular premature complexes; LVH, left ventricular hypertrophy; ST-T, ST-segment and T-wave.
Kaplan–Meier curves of the association of supraventricular premature complexes with freedom from atrial fibrillation incidence in the community-based general population. Both men and women with SVPCs had a significantly higher risk of incidence of atrial fibrillation than those without supraventricular premature complexes (the log-rank test, all P < 0.0001).
Effects of supraventricular premature complex burden on death and atrial fibrillation onset
To evaluate the effect of SVPC burden (number of SVPCs) on death and AF onset, we also calculated the HRs of each single or multiple SVPCs for death and AF incidence. In 3858 subjects with SVPCs, multiple SVPCs were recorded in 395 subjects, while 3463 had single SVPCs at baseline. Multiple SVPCs were also associated with stroke death, CVD, and all-cause death in unadjusted Cox analyses as same as single SVPCs (Supplementary Data). After adjustment for all the potential confounders, however, multiple SVPCs were not significantly associated with the increased risk of any causes of death. On the other hand, multiple SVPCs were significantly associated with the increased risk of AF onset with higher HRs than single SVPCs.
Propensity score matched analyses
We further performed propensity score matched analyses to balance the potential cardiovascular risk factors between the subjects with and without SVPCs. The baseline characteristics of the subjects who were matched using propensity score are shown in Supplementary Data. All variables were statistically comparable between groups. Supplementary Data shows the HRs of subjects with SVPCs for each endpoint. The presence of SVPCs was significantly associated with an increased risk of stroke death after adjustment for all the potential confounders for women, but not for men. The presence of SVPCs was significantly associated with an increased risk of CVD after adjustment for all the potential confounders in both men and women. On the other hands, SVPCs were not significantly associated with an increased risk of all-cause death after adjustment for all the potential confounders in both genders. Finally, the presence of SVPCs was significantly associated with an increased risk of AF development after adjustment for all the potential confounders in both men and women.
Discussion
Major findings
There were two major findings in this community-based large-scale cohort study. First, the subjects with documented SVPCs in health checkups had higher risks of death from cardiovascular causes during the 14-year follow-up. The presence of SVPCs became a significant risk for CVD after adjustment for potential cardiovascular risk factors. Additionally, SVPCs had a greater impact on cardiovascular mortality in women than in men as well as AF. Second, there was more than four-fold higher risk of AF development in subjects with SVPCs than in those without SVPCs, and the presence of SVPCs was a strong predictor of AF development among all risk factors.
Prognostic significance of supraventricular premature complexes
Supraventricular premature complexes were considered to be benign arrhythmias until the last few decades.13,14 However, the rationale of a correlation between the presence of SVPCs and an increased incidence of AF has been supported by recent advances in our understanding of the pathophysiology of AF. Haissaguerre et al.15 demonstrated that AF was triggered by SVPCs, which usually originated from the pulmonary veins. Clinical and epidemiological studies demonstrated that SVPCs were an independent electrocardiographic predictor for new onset of AF, as well as the P wave prolongation, ST-T abnormalities, and LVH.5–8,16–18 Recent prospective cohort study showed that the addition of SVPC count improved the predictive model of the Framingham AF risk algorithm.19 These previous observations clearly indicated the strong association between SVPCs and AF onset. The present study confirms the concept that SVPCs predispose subjects to development of AF, and this can be extended to a large-scale healthy general population. More notably, we demonstrated that the subjects with SVPCs, even those with only occasional single SVPCs in 12-lead ECGs, had significantly increased risk of death from cardiovascular causes even after adjustment for age and traditional cardiovascular risk factors. In addition, strengths of the present study include its population-based design, and to our best knowledge, the largest sample size. The Framingham Heart Study and the other several cohort studies have reported that AF was associated with 1.5- to 2.0-fold higher risk of CVD.20 Although the impact of SVPC on cardiovascular mortality was not as high as that of AF, the presence of SVPCs was also associated with ∼1.2- to 1.5-fold higher risk of death from cardiovascular causes. Our findings provide an awareness of the importance of the prognostic significance of SVPCs even in general population. The results of our study are consistent with several recent studies analysing standard resting 12-lead ECGs or Holter ECGs.8,21,22 However, these seemed to be in contradiction with the result of the previous study that exercise-induced SVPCs were not significantly associated with CVD.23 It has been reported that although exercise-induced SVPCs were strongly influenced by cardiac diastolic performance and exercise capacity, age was the only independent predictor of exercise-induced supraventricular arrhythmias in multivariate regression analysis.24 There may be a difference in clinical significance between SVPCs under normal condition and exercise-induced SVPCs, and the former may reflect not only age-related alteration in physiological performance but also some kind of subclinical cardiovascular abnormality.
In this study, multiple SVPCs were not linked to higher risk for death than single SVPC after adjustment for all the potential confounders. On the other hand, multiple SVPCs were significantly associated with the increased risk of AF onset with higher HRs than single SVPCs. These results suggest that the number of SVPCs even on a shortly recorded ECG was a good predictor for AF development, although the number of SVPCs on a shortly recorded ECG had a poor predictive value for death.
Mechanism underlying the association between supraventricular premature complexes and death
Although the definite reasons why the presence of SVPCs was associated with an increased risk of cardiovascular mortality remain to be fully elucidated, several considerations should be taken into account. The most likely explanation is that SVPCs trigger paroxysmal AF, followed by the development of persistent or chronic AF, and consequently result in cardiogenic thromboemboli or heart failure during the follow-up period. Alternatively, the presence of SVPCs may result from underlying asymptomatic paroxysmal AF or organic heart disease. There are wide individual differences in the symptoms of AF, and the incidence of asymptomatic AF was reported to be 10–40%.25,26 Similarly, some patients with organic heart disease are unaware of developing conditions such as silent myocardial ischaemia. In fact, the subjects with SVPCs were significantly older, and more likely to have hypertension, and LVH, ST-T abnormalities, and abnormal Q waves, which are the electrocardiographic findings strongly linked to hypertrophy or coronary heart disease, were more frequently found in the subjects with SVPCs at baseline. Therefore, it is suggested that there is a significant overlap between the risk factors of SVPCs and LVH or coronary artery disease. Recent population-based study showed that age, a history of cardiovascular disease, and a level of natriuretic peptides were strongly related with SVPC frequency.27 Therefore, SVPCs detected in health checkups may reflect some kind of subclinical cardiovascular abnormality rather than being a future predictor for the incidence of AF. On the other hands, propensity score matched analyses showed that the presence of SVPCs was significantly associated with the increased risks of AF onset and death from cardiovascular causes after adjustment for all the potential confounders in both men and women. These results suggest that the presence of SVPCs was a significant predictor for AF incidence and CVD in spite of the presence or absence of the major cardiovascular risk factors such as older age or hypertension.
Previous studies on outcomes in men and women with AF provided contradictory results. A report from Euro Heart Study indicated female patients with AF were older and more frequently had hypertension, valvular heart disease, diabetes, and thyroid disease; male patients more frequently had idiopathic AF. In total, women had more comorbidities, higher risk for stroke, and were more often symptomatic than men, which are almost consistent with the results of the Framingham study, and several clinical studies.27–31 In fact, the CHA2DS2-VASc score, a generally used score to stratify patients with AF at risk for stroke, also cited female sex as an additive risk factor for stroke. As well as AF, we showed that SVPCs had a greater impact on cardiovascular mortality and all-cause mortality in women than in men in the present study. Although the definite reason why a sex difference exists in the relationships between SVPCs and cardiovascular mortality is unclear, it may be due to more severe comorbidities associated with stroke or heart failure in female patients with SVPCs.
Study limitations
There are some limitations to this study. Since the ECG was recorded for only ∼15 s, and AF was diagnosed by only ECG, regardless of prior history, the diagnosis of SVPCs or AF may be underestimated. However, SVPCs or AF detected in such a short time might have to be regarded as clinically significant signs, rather than accidental findings. In addition, the effects of SVPC burden (number of SVPCs) on death could not be exactly evaluated because of a shortly recorded ECG. The medical history and prescribed medications were self-reported. Thus, detailed information about the past medical history or therapeutic regimen was lacking, and the participants with heart diseases might not be completely excluded in this study. The study population included more women than men. Moreover, the study participants were almost all Japanese living in a certain region. Therefore, our results might not be generalizable to other racial populations. Further studies including various different races and areas are warranted to clarify the generalizability of the present study.
Conclusions
In conclusion, the presence of SVPCs in standard resting 12-lead ECGs is associated with the increased risk of death from cardiovascular causes even after adjustment for potentially confounding variables in general population. Moreover, subjects with SVPCs have a significant risk of new onset of AF. For such a population, screening for latent heart disease or paroxysmal AF, careful medical follow-ups, and modification of risk factors, e.g. hypertension, should be encouraged. Further studies are required to identify whether and how subjects with SVPCs should be treated to improve their cardiovascular outcome.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by a Grant-in-Aid from the Ministry of Health, Labour and Welfare, and Health and Labour Sciences Research Grants, Japan [Research on Health Services: H17-Kenkou-007, Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H18-Junkankitou(Seishuu)-Ippan-012, Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H20-Junkankitou(Seishuu)-Ippan-013, Intractable Diseases Conquest Research: H21-Nanchi-Ippan-059, Intractable Diseases Conquest Research: H22-Nanchi-Ippan-144, and Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H23-Junkankitou(Seishuu)-Ippan-005].
Conflict of interest: none declared.