-
PDF
- Split View
-
Views
-
Cite
Cite
Franz H. Messerli, Sripal Bangalore, Frank Ruschitzka, Angiotensin receptor blockers: baseline therapy in hypertension?, European Heart Journal, Volume 30, Issue 20, October 2009, Pages 2427–2430, https://doi.org/10.1093/eurheartj/ehp364
Close - Share Icon Share
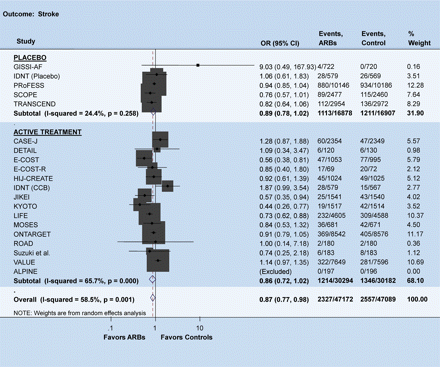
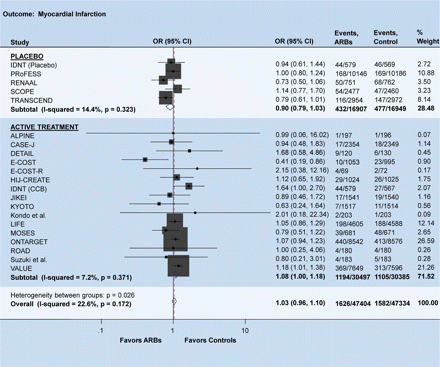
The provocative editorial by Verma and Strauss in the BMJ 5 years ago1 stating that angiotensin receptor blockers (ARBs) ‘may increase myocardial infarction—and patients may need to be told’ caused a tempest in the teapot and led to extensive scrutiny of outcome data with this drug class. In 2007 the blood pressure-lowering treatment trialists collaboration found that there were similar blood pressure-dependent effects of angiotensin-converting enzyme (ACE) inhibitors and ARBs for the risk of stroke, coronary heart disease, and heart failure.2 The authors cautioned, however, that only for ACE inhibitors but not for ARBs, was there evidence of a blood pressure-independent effect on the risk of major coronary disease events. Also, ONTARGET, a thorough, double-blind prospective randomized trial, documented equal outcome efficacy of an ARB and an ACE inhibitor in a high-risk population, although there was a trend for better stroke prevention in the ARB (telmisartan) arm and better coronary artery disease prevention in the ACE inhibitor (ramipril) arm.3 When analysing these studies and including the most recent ARB trials such as TRANSCEND,4 PRoFESS,5 CASE-J trial,6 HIJ-CREATE,7 JIKEI,8 and KYOTO,9 we found, in a database of ∼100 000 patients from 26 randomized non-heart failure trials of ARBs, a 13% reduction in the risk of stroke (P = 0.022) (Figure 1) but a trend towards increased risk of myocardial infarction, especially when compared with active treatment (P = 0.06) (Figure 2).
Odds ratio for stroke. There was a significant reduction in the risk of stroke with ARBs compared with controls. The size of the markers represents the weight of each trial. Meta-analysis was performed using the search terms ‘angiotensin receptor blockers’ with the inclusion criteria of being a randomized comparison with follow-up for at least 1 year, enrolling non-heart failure patients and evaluating outcomes of interest.
Odds ratio for myocardial infarction. There was a trend towards increased risk of myocardial infarction with ARBs compared with the active treatment group. The size of the markers represents the weight of each trial.
Much ink has been wasted on the outcome of TRANSCEND and PRoFESS, in both of which telmisartan, despite a fall in blood pressure, did not reduce cardiovascular events better than placebo. We perhaps also should consider that a majority of patients in both of these trials were pre-treated with a blocker of the renin–angiotensin system (RAS). Thus, both trials were evaluating the effects of discontinuation of RAS blockade rather than the effects of starting RAS blockade. Conceivably we are observing, in both of these studies, a ‘legacy effect’ of pre-randomization RAS blockade. Also, we should not forget that several ACE inhibitor trials such as QUIET,10 PEACE,11 PROGRESS,12 and CAMELOT13 did not beat placebo despite a significant fall in blood pressure.
For once, in the present KYOTO study, there was no significant difference in blood pressure between the two treatment arms. At study end, ∼50% more patients were treated with a calcium antagonist in the non-ARB arm. Thus, KYOTO is somewhat reminiscent of VALUE14 in which patients were, in a double-blind prospective study design, randomized to either valsartan or amlodipine. However, the results of KYOTO are diametrically different from those of VALUE in that KYOTO, for a given blood pressure reduction, showed a 45% reduction of the primary endpoint compared with the non-ARB arm. This benefit was driven mainly by stroke and, somewhat surprisingly, angina, while there was no significant difference in myocardial infarction, heart failure, and all-cause mortality. In contrast, in VALUE, stroke, if anything, was reduced less well in the valsartan arm than in the amlodipine arm. While the disparity in blood pressure control in favour of amlodipine may have confounded the interpretation of the results of the (double-blind) VALUE trial, in (open label) KYOTO, despite the difference in treatment options, surprisingly exactly the same blood pressure reduction was achieved in the ARB and non-ARB arm throughout the study. Similar to VALUE,14 the risk of new-onset diabetes was significantly less in the valsartan than in the non-ARB arm.
Could the different results of VALUE and KYOTO be explained by the study populations? Asians may be particularly receptive to the protective effects of ARBs, as was shown in RENAAL15 where most of the benefits occurred in the Asian subpopulation. Cerebrovascular disease is more prevalent and coronary artery disease less prevalent in Asians than in western societies. Not surprisingly, therefore, a reduction of strokes is easier to document than a reduction of coronary artery disease, as was shown in both the JIKEI-Heart Study and KYOTO. With regard to angina, we should remember that this is a rather soft endpoint and that because of the open label design, investigators were fully aware of the treatment being administered. We find it somewhat difficult to believe that an ARB should be better in preventing angina than a calcium antagonist, as was observed in both KYOTO and the JIKEI-Heart Study.
In contrast to JIKEI and KYOTO, in the other two similarly designed ARB studies in the Asian population, i.e. CASE-J and HIJ-CREATE, ARB treatment with candesartan did not reduce morbidity and mortality better than non-ARB therapy. Whether the difference in outcome between the two valsartan and the two candesartan studies is due to chance or to differences in the ARB molecule, in study design, in the patient population, or in the concomitant medication cannot be determined.
In KYOTO, adverse events were uncommon and medication was well tolerated. This is not surprising since many studies have documented that ARBs are exceedingly well tolerated. In fact, in a few studies in which ARBs were compared with a placebo, patients on ARB therapy had significantly fewer headaches than those on placebo. In a thorough Cochrane meta-analysis, withdrawal rates due to adverse effects in a total of 46 studies and 13 451 patients were significantly lower on ARBs than on placebo [RR 0.68 (95% CI 0.54–0.87)].16 In contrast, the Cochrane meta-analysis of 92 studies and 12 954 patients documented that ACE inhibitors are not better tolerated than placebo [RR 0.85(95% CI 0.67–1.07)].17
The low withdrawal rates in the ARB arm would indicate that hypertension is perhaps not necessarily such an asymptomatic disease as we thought and taught. Low-grade headache, fatigue, and other non-specific symptoms may be associated with sustained blood pressure elevation. A reduction of blood pressure by an ARB or an ACE inhibitor may reduce such low-grade symptoms better than does placebo. However, the inherent adverse effects of the ACE inhibitor (cough and, uncommonly, angioedema) seem to abolish the overall achieved benefit. Indeed, head to head comparisons suggest a better tolerability of ARBs than of ACE inhibitors.18
The impressive results of the KYOTO study lead to the question of whether ARBs as a class have come of age and should now be considered as preferred or baseline therapy in hypertension. The answer, with regard to safety and efficacy, could be a resounding yes, i.e. if efficacy were defined as blood pressure reduction. However, blood pressure is merely a surrogate endpoint which correlates to some extent with the true endpoint, i.e. heart attack, stroke, and death. As the above meta-analysis demonstrates, ARBs are efficacious and even superior to other drug classes in stroke prevention but their efficacy with regard to coronary events remains uncertain. Thus, if efficacy is defined as reduction in overall cardiovascular events and mortality, the answer, in view of the data in aggregate, remains no, or perhaps, not yet.
Conflict of interest: none declared.
References
doi:10.1093/eurheartj/ehp363
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.