-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Valgimigli, Giuseppe Biondi-Zoccai, Matteo Tebaldi, Arnoud W.J. van 't Hof, Gianluca Campo, Christian Hamm, Jurriën ten Berg, Leonardo Bolognese, Francesco Saia, Gian Battista Danzi, Carlo Briguori, Ertan Okmen, Spencer B. King, David J. Moliterno, Eric J. Topol, Tirofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: a meta-analysis of randomized trials, European Heart Journal, Volume 31, Issue 1, January 2010, Pages 35–49, https://doi.org/10.1093/eurheartj/ehp376
Close - Share Icon Share
Abstract
To perform a thorough and updated systematic review of randomized clinical trials comparing tirofiban vs. placebo or vs. abciximab.
We searched for randomized trials comparing tirofiban vs. placebo or any active control. Odds ratios (OR) were computed from individual studies and pooled with random-effect methods. Thirty-one studies were identified involving 20 006 patients (12 874 comparing tirofiban vs. heparin plus placebo or bivalirudin alone, and 7132 vs. abciximab). When compared with placebo, tirofiban was associated at 30 days with a significant reduction in mortality [OR = 0.68 (0.54–0.86); P = 0.001] and death or myocardial infarction (MI) [OR = 0.69 (0.58–0.81); P < 0.001]. The treatment benefit persisted at follow-up but came at an increased risk of minor bleedings [OR = 1.42 (1.13, 1.79), P = 0.002] or thrombocytopenia. When compared with abciximab, mortality at 30 days did not differ [OR = 0.90 (0.53, 1.54); P = 0.70], but in the overall group tirofiban trended to increase the composite of death or MI [OR = 1.18 (0.96, 1.45); P = 0.11]. No such trend persisted at medium-term follow-up or when appraising studies testing tirofiban at 25 µg/kg bolus regimen.
Tirofiban administration reduces mortality, the composite of death or MI and increases minor bleedings when compared with placebo. An early ischaemic hazard disfavouring tirofiban was noted when compared with abciximab in studies based on 10 but not 25 µg/kg tirofiban bolus regimen.
Introduction
Tirofiban is a small molecule, non-peptide tyrosine derivative which belongs to the class of glycoprotein (GP) IIb/IIIa inhibitors (GPI).1,2 By preventing the binding of fibrinogen and von Willebrand factor to the GP IIb/IIIa receptor on the surface of the platelet, GPIs are currently regarded as the most potent inhibitors of platelet aggregation.1,2
Though similar to abciximab in that it has a high affinity for the GP IIb/IIIa receptor, tirofiban dissociates from the GP IIb/IIIa receptor more rapidly than abciximab.1,2 Its anti-aggretory effects reverse within hours after the completion of the infusion, whereas abciximab binds near irreversibly to the receptor resulting in a considerably longer effect.1,3 Additionally, tirofiban does not inhibit other β3 integrins, such as the vitronectin receptor, at the surface of vascular cells or the activated MAC-1 receptor on leucocytes,4 which have been traditionally regarded as crucial targets to explain abciximab effects on microcirculation.5
Even more importantly, different dosing regimens of tirofiban have been developed over time based on the clinical setting and the timing of percutaneous coronary intervention (PCI) which has resulted in mixed results in clinical trials when compared with either placebo or abciximab.3,6–10 Thus, uncertainty on the role of tirofiban still largely persists in current clinical practice.
Systematic reviews employing meta-analytic techniques provide quantitative and objective means to pool and assess available clinical evidence, emphasizing internal validity and homogeneity, while affording increased statistical power for hypothesis testing. The aim of this study was thus to perform a thorough and updated systematic review of randomized clinical trials comparing tirofiban vs. placebo or vs. abciximab in patients undergoing treatment for various coronary artery disease (CAD) conditions, with specific emphasis on the role of front-loaded tirofiban regimen and timing of intervention.
Methods
Search strategy
Two expert cardiologists (M.V., M.T.) independently and systematically searched BioMedCentral, CENTRAL, Clinicaltrials.gov, EMBASE, and PubMed for randomized trials comparing tirofiban vs. placebo or any active control in patients with acute coronary syndromes and/or undergoing PCI (updated October 2008), with divergences resolved after consensus.11 EMBASE and PubMed were searched with explode features according to the following strategy: ‘(abciximab[all] OR tirofiban[all] OR (glycoprotein[all] AND (iib/iiia OR iibiiia) AND inhibitor*[all])) AND coronary AND (clinical trial*[all] OR random*[all])’.12
Articles published in languages other than English or Italian (the native languages of the authors) were systematically searched in multiple online databases, international conference proceedings, references (backward snowballing) or cross-quotations (forward snowballing) from included studies and pertinent available quantitative reviews, and queries to international experts. References were systematically scanned to retrieve additional studies. No language restriction was enforced.
Selection criteria
Shortlisted studies were retrieved as full articles and appraised by three unblinded reviewers independently (M.V., G.B.Z., M.T.), with divergences resolved after consensus, according to the following inclusion criteria: (i) randomized treatment allocation and (ii) comparison of tirofiban vs. placebo or active. Exclusion criteria were: (i) duplicate reports failing to report additional or extended clinical outcomes, (ii) lack of outcome data beyond hospitalization, and (iii) equivocal (i.e. no clear information on modalities for allocating patients to tirofiban vs. placebo or active treatment) or non-random treatment allocation.
Data abstraction and validity assessment
Three reviewers independently abstracted data, with divergences resolved after consensus. In case of incomplete or unclear data, authors were contacted where possible. The co-primary endpoints of the analysis were the 30 days and long-term mortality rates. The incidence of major adverse cardiovascular events (MACE), including the composite of death, myocardial infarction (MI), or urgent revascularization, death or MI, as well as major and minor bleeding [according to the Thrombolysis In Myocardial Infarction (TIMI) criteria]13 and thrombocytopenia were also appraised. We pre hoc stratified studies according to the type of control, dosage/timing of tirofiban administration, and type of concomitant oral anti-platelet therapy. Thus, studies where more than one dosage or timing of intervention were tested vs. placebo or active control have been split up into the most suitable pre-specified study categories. Additional pertinent data for baseline and procedural characteristics were abstracted, including type of PCI. Study validity and risk of bias were appraised according to The Cochrane Collaboration methods, i.e. separately appraising means for generating the randomization sequence, allocation concealment, blinding, concurrent treatment, data completion, definitions, outcome reporting, other potential source of bias, and overall risk of bias.11
Data analysis and synthesis
Odds ratios (OR) were computed from individual studies and pooled according to DerSimonian-Laird random-effect methods (with 95% confidence intervals) using RevMan 4.2 (The Cochrane Collaboration, Købehavn, Denmark). Inconsistency was appraised by means of I2. Specifically, I2 < 25% suggests mild, statistical inconsistency, whereas I2 values in the 25–50% range and in the 75−100% represent, respectively, moderate and extensive inconsistency. Statistical heterogeneity was appraised with χ2 tests, with P-values less than 0.10 suggesting underlying heterogeneity. Small study bias and/or publication bias (i.e. the likelihood of small yet nominally significant studies being selectively published in the literature) were appraised by means of visual inspection of funnel plots and Peters test.14 Random-effect meta-regression was also performed to explore moderators and/or predictors of changes in log-transformed OR, by means of a weighted-least-square inverse-variance weighted method with SPSS 11.0 (SPSS, Chicago, IL, USA). Unadjusted P-values are reported throughout, with hypothesis testing set at the two-tailed 0.05 level.
According to absolute risk reduction or increment obtained with random-effect risk differences computed at 30-day follow-up, we calculated the number needed to treat (NNT) to prevent one event, whereas for the safety endpoints, we calculated the number needed to harm (NNH) to determine one adverse event.
Results
Search results and study selection
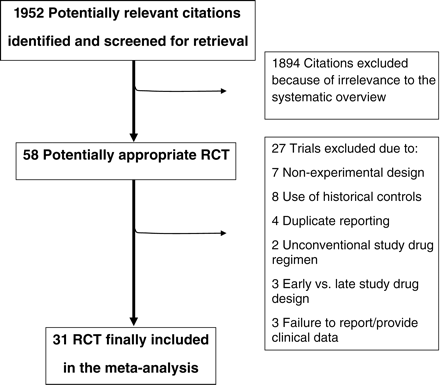
Database searches retrieved 1952 citations (Figure 1). Shortlisted citations were retrieved and checked at the title/abstract level excluding 1894 papers. Complete articles for the remaining 58 studies were checked for compliance to inclusion/exclusion criteria. Reasons for further exclusion included non-experimental design, use of historical controls, duplicate reporting, unconventional study drug regimen, early vs. late study drug administration design, or failure to report/provide upon request clinical data. We finally identified 31 eligible trials of which 22 were controlled with placebo6–8,15–35 (Table 1), eight with abciximab,9,10,36–41 and one with both agents42 (Table 2).
Flow diagram of the systematic literature search indicating the inclusion and exclusion process of studies.
Main characteristics of the placebo-controlled trials
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | 2006–2008 | 263 | ASA and/or clopidogrel poor responders | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH or bivalirudin, and clopidogrel | Periprocedural MI defined as troponin >3× ULN within 48 h | 30 Days |
| ADVANCE25 | 2002–2003 | 202 | High-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine or clopidogrel | Death, MI, TVR, or bailout tirofiban | 6 Months |
| ELISA 229 | 2002–2005 | 328 | NSTEACS | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, LWMH, clopdidogrel | Enzymatic infarct size (LDHQ 48) | 30 Days |
| Ercan et al.22 | Not reported | 57 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Levels of C-reactive protein at 48 h | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Platelet aggregation inhibition | Hospital stay |
| Fu et al.30 | 2005–2007 | 150 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Not reported | 30 Days |
| Ivandic et al.31 | 2004–2006 | 100 | NSTEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Infarct size based on troponin T elevation | 6 Months |
| Juergens et al.18 | Not reported | 894 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | TIMI bleedings | 30 Days |
| Kereiakes et al.15 | Not reported | 44 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Platelet aggregation inhibition | Hospital stay |
| Kim et al.26 | 2001–2002 | 160 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH or dalteparin, | Not reported | 6 Months |
| Kurowski et al.27 | 2000–2003 | 50 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Myocardial necrosis as evidenced by an increase in the cTnT above the ULN within 72 h | 21 Months |
| NAPLES34 | 2005–2008 | 335 | Elective PCI in diabetics | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | NACE | 30 Days |
| Okmen et al.21 | Not reported | 83 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Infarct size based on CK-MB >2× ULN | 10 Months |
| Okmen et al.24 | Not reported | 119 | Stable or unstable angina undergoing PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, ticlopidine, UFH | Infarct size based on CK-MB >2× ULN | 21 Months |
| On-TIME 2 open-label study33 | 2004–2006 | 414 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | None | 30 Days |
| On-TIME 233 | 2006–2007 | 984 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Residual ST-segment deviation at ECG | 30 Days |
| Ozkan et al.28 | 1999–2004 | 47 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, enoxaparin, and clopidogrel | Not reported | 30 Days |
| PRISM8 | 1994–1996 | 3232 | NSTEACS | Bolus: 0.6 µg/kg/min×30 min; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH in placebo group only | Death, MI, refractory ischemia at 48 h | 30 Days |
| PRISM-PLUS7 | 1994–1996 | 1570 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Death, MI, refractory ischemia at 7 days | 6 Months |
| RESTORE6 | 1995 | 2212 | NSTEACS or STEMI undergoing POBA or DCA | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Death, MI, TVR, or bailout stenting | 6 Months |
| SASTRE23 | 2000–2002 | 144 | STEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, alteplase | TIMI flow grade 3 in the IRA at 90′ | 30 Days |
| Shen et al.32 | 2005–2006 | 115 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | MACE at 30 days | 6 Months |
| TETAMI19,20 | 1999–2002 | 1224 | STEMI ineligible to lysis | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, enoxaparin or UFH | Death, MI, recurrent angina | 30 Days |
| TOPSTAR17 | Not reported | 96 | Stable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Troponin elevation within 48 h | 9 Months |
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | 2006–2008 | 263 | ASA and/or clopidogrel poor responders | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH or bivalirudin, and clopidogrel | Periprocedural MI defined as troponin >3× ULN within 48 h | 30 Days |
| ADVANCE25 | 2002–2003 | 202 | High-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine or clopidogrel | Death, MI, TVR, or bailout tirofiban | 6 Months |
| ELISA 229 | 2002–2005 | 328 | NSTEACS | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, LWMH, clopdidogrel | Enzymatic infarct size (LDHQ 48) | 30 Days |
| Ercan et al.22 | Not reported | 57 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Levels of C-reactive protein at 48 h | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Platelet aggregation inhibition | Hospital stay |
| Fu et al.30 | 2005–2007 | 150 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Not reported | 30 Days |
| Ivandic et al.31 | 2004–2006 | 100 | NSTEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Infarct size based on troponin T elevation | 6 Months |
| Juergens et al.18 | Not reported | 894 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | TIMI bleedings | 30 Days |
| Kereiakes et al.15 | Not reported | 44 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Platelet aggregation inhibition | Hospital stay |
| Kim et al.26 | 2001–2002 | 160 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH or dalteparin, | Not reported | 6 Months |
| Kurowski et al.27 | 2000–2003 | 50 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Myocardial necrosis as evidenced by an increase in the cTnT above the ULN within 72 h | 21 Months |
| NAPLES34 | 2005–2008 | 335 | Elective PCI in diabetics | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | NACE | 30 Days |
| Okmen et al.21 | Not reported | 83 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Infarct size based on CK-MB >2× ULN | 10 Months |
| Okmen et al.24 | Not reported | 119 | Stable or unstable angina undergoing PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, ticlopidine, UFH | Infarct size based on CK-MB >2× ULN | 21 Months |
| On-TIME 2 open-label study33 | 2004–2006 | 414 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | None | 30 Days |
| On-TIME 233 | 2006–2007 | 984 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Residual ST-segment deviation at ECG | 30 Days |
| Ozkan et al.28 | 1999–2004 | 47 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, enoxaparin, and clopidogrel | Not reported | 30 Days |
| PRISM8 | 1994–1996 | 3232 | NSTEACS | Bolus: 0.6 µg/kg/min×30 min; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH in placebo group only | Death, MI, refractory ischemia at 48 h | 30 Days |
| PRISM-PLUS7 | 1994–1996 | 1570 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Death, MI, refractory ischemia at 7 days | 6 Months |
| RESTORE6 | 1995 | 2212 | NSTEACS or STEMI undergoing POBA or DCA | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Death, MI, TVR, or bailout stenting | 6 Months |
| SASTRE23 | 2000–2002 | 144 | STEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, alteplase | TIMI flow grade 3 in the IRA at 90′ | 30 Days |
| Shen et al.32 | 2005–2006 | 115 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | MACE at 30 days | 6 Months |
| TETAMI19,20 | 1999–2002 | 1224 | STEMI ineligible to lysis | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, enoxaparin or UFH | Death, MI, recurrent angina | 30 Days |
| TOPSTAR17 | Not reported | 96 | Stable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Troponin elevation within 48 h | 9 Months |
ASA, aspirin; LWMH, low-weight molecular heparin; LDH, lactate dehydrogenase; NSTEACS, non-ST-segment elevation acute coronary syndromes; NSTEMI, non-ST-segment elevation myocardial infarction; MI, myocardial infarction; LDHQ48, area under the lactate dehydrogenase release over 48 h curve; POBA, balloon angioplasty; DCA, directional coronary atherectomy; STEMI, ST-segment elevation myocardial infarction; ULN, upper limit of normal; PCI, percutaneous coronary intervention; SVG, saphenous vein graft; IRA, infarct-related artery; MACE, major adverse cardiovascular events; NACE, net adverse cardiovascular events; UFH, unfractionated heparin; TVR, target vessel revascularization.
Main characteristics of the placebo-controlled trials
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | 2006–2008 | 263 | ASA and/or clopidogrel poor responders | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH or bivalirudin, and clopidogrel | Periprocedural MI defined as troponin >3× ULN within 48 h | 30 Days |
| ADVANCE25 | 2002–2003 | 202 | High-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine or clopidogrel | Death, MI, TVR, or bailout tirofiban | 6 Months |
| ELISA 229 | 2002–2005 | 328 | NSTEACS | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, LWMH, clopdidogrel | Enzymatic infarct size (LDHQ 48) | 30 Days |
| Ercan et al.22 | Not reported | 57 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Levels of C-reactive protein at 48 h | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Platelet aggregation inhibition | Hospital stay |
| Fu et al.30 | 2005–2007 | 150 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Not reported | 30 Days |
| Ivandic et al.31 | 2004–2006 | 100 | NSTEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Infarct size based on troponin T elevation | 6 Months |
| Juergens et al.18 | Not reported | 894 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | TIMI bleedings | 30 Days |
| Kereiakes et al.15 | Not reported | 44 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Platelet aggregation inhibition | Hospital stay |
| Kim et al.26 | 2001–2002 | 160 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH or dalteparin, | Not reported | 6 Months |
| Kurowski et al.27 | 2000–2003 | 50 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Myocardial necrosis as evidenced by an increase in the cTnT above the ULN within 72 h | 21 Months |
| NAPLES34 | 2005–2008 | 335 | Elective PCI in diabetics | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | NACE | 30 Days |
| Okmen et al.21 | Not reported | 83 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Infarct size based on CK-MB >2× ULN | 10 Months |
| Okmen et al.24 | Not reported | 119 | Stable or unstable angina undergoing PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, ticlopidine, UFH | Infarct size based on CK-MB >2× ULN | 21 Months |
| On-TIME 2 open-label study33 | 2004–2006 | 414 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | None | 30 Days |
| On-TIME 233 | 2006–2007 | 984 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Residual ST-segment deviation at ECG | 30 Days |
| Ozkan et al.28 | 1999–2004 | 47 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, enoxaparin, and clopidogrel | Not reported | 30 Days |
| PRISM8 | 1994–1996 | 3232 | NSTEACS | Bolus: 0.6 µg/kg/min×30 min; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH in placebo group only | Death, MI, refractory ischemia at 48 h | 30 Days |
| PRISM-PLUS7 | 1994–1996 | 1570 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Death, MI, refractory ischemia at 7 days | 6 Months |
| RESTORE6 | 1995 | 2212 | NSTEACS or STEMI undergoing POBA or DCA | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Death, MI, TVR, or bailout stenting | 6 Months |
| SASTRE23 | 2000–2002 | 144 | STEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, alteplase | TIMI flow grade 3 in the IRA at 90′ | 30 Days |
| Shen et al.32 | 2005–2006 | 115 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | MACE at 30 days | 6 Months |
| TETAMI19,20 | 1999–2002 | 1224 | STEMI ineligible to lysis | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, enoxaparin or UFH | Death, MI, recurrent angina | 30 Days |
| TOPSTAR17 | Not reported | 96 | Stable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Troponin elevation within 48 h | 9 Months |
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | 2006–2008 | 263 | ASA and/or clopidogrel poor responders | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH or bivalirudin, and clopidogrel | Periprocedural MI defined as troponin >3× ULN within 48 h | 30 Days |
| ADVANCE25 | 2002–2003 | 202 | High-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine or clopidogrel | Death, MI, TVR, or bailout tirofiban | 6 Months |
| ELISA 229 | 2002–2005 | 328 | NSTEACS | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, LWMH, clopdidogrel | Enzymatic infarct size (LDHQ 48) | 30 Days |
| Ercan et al.22 | Not reported | 57 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Levels of C-reactive protein at 48 h | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Platelet aggregation inhibition | Hospital stay |
| Fu et al.30 | 2005–2007 | 150 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Not reported | 30 Days |
| Ivandic et al.31 | 2004–2006 | 100 | NSTEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Infarct size based on troponin T elevation | 6 Months |
| Juergens et al.18 | Not reported | 894 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | TIMI bleedings | 30 Days |
| Kereiakes et al.15 | Not reported | 44 | Elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Platelet aggregation inhibition | Hospital stay |
| Kim et al.26 | 2001–2002 | 160 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH or dalteparin, | Not reported | 6 Months |
| Kurowski et al.27 | 2000–2003 | 50 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Myocardial necrosis as evidenced by an increase in the cTnT above the ULN within 72 h | 21 Months |
| NAPLES34 | 2005–2008 | 335 | Elective PCI in diabetics | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | NACE | 30 Days |
| Okmen et al.21 | Not reported | 83 | NSTEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Infarct size based on CK-MB >2× ULN | 10 Months |
| Okmen et al.24 | Not reported | 119 | Stable or unstable angina undergoing PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, ticlopidine, UFH | Infarct size based on CK-MB >2× ULN | 21 Months |
| On-TIME 2 open-label study33 | 2004–2006 | 414 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | None | 30 Days |
| On-TIME 233 | 2006–2007 | 984 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | Residual ST-segment deviation at ECG | 30 Days |
| Ozkan et al.28 | 1999–2004 | 47 | Stable or marker-negative unstable angina undergoing SVG stenting | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, enoxaparin, and clopidogrel | Not reported | 30 Days |
| PRISM8 | 1994–1996 | 3232 | NSTEACS | Bolus: 0.6 µg/kg/min×30 min; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH in placebo group only | Death, MI, refractory ischemia at 48 h | 30 Days |
| PRISM-PLUS7 | 1994–1996 | 1570 | NSTEACS | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH | Death, MI, refractory ischemia at 7 days | 6 Months |
| RESTORE6 | 1995 | 2212 | NSTEACS or STEMI undergoing POBA or DCA | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Death, MI, TVR, or bailout stenting | 6 Months |
| SASTRE23 | 2000–2002 | 144 | STEMI | Bolus: 0.4 µg/kg/min×30 min; infusion: 0.1 µg/kg/min | Upstream | ASA, UFH, alteplase | TIMI flow grade 3 in the IRA at 90′ | 30 Days |
| Shen et al.32 | 2005–2006 | 115 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, UFH, clopidogrel | MACE at 30 days | 6 Months |
| TETAMI19,20 | 1999–2002 | 1224 | STEMI ineligible to lysis | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Upstream | ASA, enoxaparin or UFH | Death, MI, recurrent angina | 30 Days |
| TOPSTAR17 | Not reported | 96 | Stable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Troponin elevation within 48 h | 9 Months |
ASA, aspirin; LWMH, low-weight molecular heparin; LDH, lactate dehydrogenase; NSTEACS, non-ST-segment elevation acute coronary syndromes; NSTEMI, non-ST-segment elevation myocardial infarction; MI, myocardial infarction; LDHQ48, area under the lactate dehydrogenase release over 48 h curve; POBA, balloon angioplasty; DCA, directional coronary atherectomy; STEMI, ST-segment elevation myocardial infarction; ULN, upper limit of normal; PCI, percutaneous coronary intervention; SVG, saphenous vein graft; IRA, infarct-related artery; MACE, major adverse cardiovascular events; NACE, net adverse cardiovascular events; UFH, unfractionated heparin; TVR, target vessel revascularization.
Main characteristics of the abciximab-controlled trials
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| Danzi et al.37 | 2002 | 100 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Infarct-zone wall motion score index at 30 days | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, Clopidogrel | Platelet aggregation inhibition | Hospital stay |
| EVEREST36 | 2003–2004 | 61 | NSTEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | TIMI myocardial perfusion rate | 30 Days |
| FATA38 | 2004–2007 | 692 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | ≥70% STR at 90′ | 30 Days |
| MULTISTRATEGY10 | 2004–2007 | 744 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | ≥50% STR at 90′ | 8 Months |
| Neumann et al.40 | Not reported | 40 | Stable or unstable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine | Inhibition of platelet aggregation after 2 h of infusion | 30 Days |
| STRATEGY41 | 2003–2004 | 175 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, stroke, binary restenosis | 36 Months |
| TARGET9 | 1999–2000 | 5308 | Urgent and elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR at 30 days | 12 Months |
| TENACITY43 | 2004–2005 | 383 | Medium to high-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR | 30 Days |
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| Danzi et al.37 | 2002 | 100 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Infarct-zone wall motion score index at 30 days | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, Clopidogrel | Platelet aggregation inhibition | Hospital stay |
| EVEREST36 | 2003–2004 | 61 | NSTEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | TIMI myocardial perfusion rate | 30 Days |
| FATA38 | 2004–2007 | 692 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | ≥70% STR at 90′ | 30 Days |
| MULTISTRATEGY10 | 2004–2007 | 744 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | ≥50% STR at 90′ | 8 Months |
| Neumann et al.40 | Not reported | 40 | Stable or unstable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine | Inhibition of platelet aggregation after 2 h of infusion | 30 Days |
| STRATEGY41 | 2003–2004 | 175 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, stroke, binary restenosis | 36 Months |
| TARGET9 | 1999–2000 | 5308 | Urgent and elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR at 30 days | 12 Months |
| TENACITY43 | 2004–2005 | 383 | Medium to high-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR | 30 Days |
ASA, aspirin; UFH, unfractionated heparin; NSTEMI, non-ST-segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; MI, myocardial infarction; TVR, target vessel revascularization; STR, ST-segment resolution.
Main characteristics of the abciximab-controlled trials
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| Danzi et al.37 | 2002 | 100 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Infarct-zone wall motion score index at 30 days | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, Clopidogrel | Platelet aggregation inhibition | Hospital stay |
| EVEREST36 | 2003–2004 | 61 | NSTEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | TIMI myocardial perfusion rate | 30 Days |
| FATA38 | 2004–2007 | 692 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | ≥70% STR at 90′ | 30 Days |
| MULTISTRATEGY10 | 2004–2007 | 744 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | ≥50% STR at 90′ | 8 Months |
| Neumann et al.40 | Not reported | 40 | Stable or unstable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine | Inhibition of platelet aggregation after 2 h of infusion | 30 Days |
| STRATEGY41 | 2003–2004 | 175 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, stroke, binary restenosis | 36 Months |
| TARGET9 | 1999–2000 | 5308 | Urgent and elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR at 30 days | 12 Months |
| TENACITY43 | 2004–2005 | 383 | Medium to high-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR | 30 Days |
| Study . | Trial period . | No. randomized . | Patient population . | Tirofiban dose . | Setting . | Concomitant anti-thrombotics . | 1° Endpoint . | Follow-up . |
|---|---|---|---|---|---|---|---|---|
| Danzi et al.37 | 2002 | 100 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | Infarct-zone wall motion score index at 30 days | 30 Days |
| Ernst et al.42 | 2002–2003 | 60 | STEMI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, Clopidogrel | Platelet aggregation inhibition | Hospital stay |
| EVEREST36 | 2003–2004 | 61 | NSTEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | TIMI myocardial perfusion rate | 30 Days |
| FATA38 | 2004–2007 | 692 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH | ≥70% STR at 90′ | 30 Days |
| MULTISTRATEGY10 | 2004–2007 | 744 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | ≥50% STR at 90′ | 8 Months |
| Neumann et al.40 | Not reported | 40 | Stable or unstable angina | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, ticlopidine | Inhibition of platelet aggregation after 2 h of infusion | 30 Days |
| STRATEGY41 | 2003–2004 | 175 | STEMI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, stroke, binary restenosis | 36 Months |
| TARGET9 | 1999–2000 | 5308 | Urgent and elective PCI | Bolus: 10 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR at 30 days | 12 Months |
| TENACITY43 | 2004–2005 | 383 | Medium to high-risk PCI | Bolus: 25 µg/kg; infusion: 0.15 µg/kg/min | Downstream | ASA, UFH, clopidogrel | Death, MI, urgent TVR | 30 Days |
ASA, aspirin; UFH, unfractionated heparin; NSTEMI, non-ST-segment elevation myocardial infarction; MI, myocardial infarction; PCI, percutaneous coronary intervention; MI, myocardial infarction; TVR, target vessel revascularization; STR, ST-segment resolution.
Study and patient characteristics
The 31 studies included in the final analysis 20 006 randomized patients (average follow-up 5 months), 12 874 vs. placebo and 7132 vs. abciximab. In seven placebo-controlled trials,7,8,21–23,26,28 mainly recruiting patients with confirmed or suspected non-ST-segment elevation acute coronary syndrome (NSTEACS) or ST-elevation myocardial infarction (STEMI), tirofiban was co-administered together with unfractioned heparin (UFH) upon presentation (upstream) as a 0.4 µg/kg 30 min bolus regimen followed by 0.1 µg/kg/min infusion apart from PRISM study,8 where tirofiban was given without UFH at a 0.6 µg/kg 30 min bolus regimen followed by 0.15 µg/kg/min infusion.
In five placebo-controlled studies,19,20,27,29,31,32 tirofiban was administered upstream in NSTEACS, STEMI, or stable CAD patients at 10 µg/kg 3 min bolus regimen and 0.15 µg/kg/min infusion, whereas in 12 studies, eight placebo-controlled,6,15,17,18,24,30,32,34 two abciximab-controlled,9,39,40 one controlled with both agents,42 and one with bivalirudin,34 tirofiban was given at 10 µg/kg 3 min bolus regimen and 0.15 µg/kg/min infusion prior PCI (downstream) in patients with stable or unstable CAD. Finally, in 4076 patients tirofiban was administered downstream at high bolus dose (25 µg/kg 3 min bolus regimen and 0.15 µg/kg/min infusion) and controlled with placebo (n = 1863)25,33,35,42 or abciximab (n = 2213).10,36–38,41–43 Study quality and risk of bias were variable, reflecting heterogeneity in setting, sample size, and study design (phase III vs. IV) and is detailed in Table 3.
Risk of bias assessment
| Study . | Adequate sequence generation . | Allocation concealment used . | Blinding . | Concurrent therapies similar . | Incomplete outcome data addressed . | Uniform and explicit outcome definitions . | Free of selective outcome reporting . | Free of other bias . | Overall risk of bias . |
|---|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | Yes (computer generated) | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| ADVANCE25 | Yes (computer generated) | Yes (external personnel) | Yes (patients, caring physicians, and outcome assessors) | Yes | No | Yes | Yes | Yes | Low |
| Danzi et al.37 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| ELISA 229 | Unclear | Unclear | Yes (outcome assessors) | No | Yes | Yes | Yes | Yes | Moderate |
| Ercan et al.22 | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | Moderate |
| Ernst et al.42 | Yes (computer generated) | Unclear | No | Yes | No | Yes | Yes | Yes | Moderate |
| EVEREST36 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| FATA38 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Fu et al.30 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Ivandic et al.31 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Juergens et al.18 | Unclear | Unclear | Yes (patients and caring physicians) | No | Yes | Yes | Yes | Yes | Moderate |
| Kereiakes et al.15 | Unclear | Unclear | Yes (patients and caring physicians) | Yes | No | No | No | Yes | Moderate |
| Kim et al.26 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Kurowski et al.27 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| MULTISTRATEGY10 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| NAPLES34 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Neumann et al.40 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Okmen et al.21 | Unclear | Unclear | No | Unclear | No | No | Yes | Yes | Moderate |
| Okmen et al.24 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| On-TIME 2 open-label study33 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| On-TIME 233 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Ozkan et al.28 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| PRISM8 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| PRISM-PLUS7 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| RESTORE6 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| SASTRE23 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| Shen et al.32 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| STRATEGY41 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Moderate |
| TARGET9 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TENACITY43 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TETAMI19,20 | Unclear | Unclear | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TOPSTAR17 | Unclear | Yes (external personnel) | Yes (patients and caring physicians) | Yes | Yes | No | No | Yes | Moderate |
| Study . | Adequate sequence generation . | Allocation concealment used . | Blinding . | Concurrent therapies similar . | Incomplete outcome data addressed . | Uniform and explicit outcome definitions . | Free of selective outcome reporting . | Free of other bias . | Overall risk of bias . |
|---|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | Yes (computer generated) | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| ADVANCE25 | Yes (computer generated) | Yes (external personnel) | Yes (patients, caring physicians, and outcome assessors) | Yes | No | Yes | Yes | Yes | Low |
| Danzi et al.37 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| ELISA 229 | Unclear | Unclear | Yes (outcome assessors) | No | Yes | Yes | Yes | Yes | Moderate |
| Ercan et al.22 | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | Moderate |
| Ernst et al.42 | Yes (computer generated) | Unclear | No | Yes | No | Yes | Yes | Yes | Moderate |
| EVEREST36 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| FATA38 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Fu et al.30 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Ivandic et al.31 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Juergens et al.18 | Unclear | Unclear | Yes (patients and caring physicians) | No | Yes | Yes | Yes | Yes | Moderate |
| Kereiakes et al.15 | Unclear | Unclear | Yes (patients and caring physicians) | Yes | No | No | No | Yes | Moderate |
| Kim et al.26 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Kurowski et al.27 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| MULTISTRATEGY10 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| NAPLES34 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Neumann et al.40 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Okmen et al.21 | Unclear | Unclear | No | Unclear | No | No | Yes | Yes | Moderate |
| Okmen et al.24 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| On-TIME 2 open-label study33 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| On-TIME 233 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Ozkan et al.28 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| PRISM8 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| PRISM-PLUS7 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| RESTORE6 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| SASTRE23 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| Shen et al.32 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| STRATEGY41 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Moderate |
| TARGET9 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TENACITY43 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TETAMI19,20 | Unclear | Unclear | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TOPSTAR17 | Unclear | Yes (external personnel) | Yes (patients and caring physicians) | Yes | Yes | No | No | Yes | Moderate |
Risk of bias assessment
| Study . | Adequate sequence generation . | Allocation concealment used . | Blinding . | Concurrent therapies similar . | Incomplete outcome data addressed . | Uniform and explicit outcome definitions . | Free of selective outcome reporting . | Free of other bias . | Overall risk of bias . |
|---|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | Yes (computer generated) | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| ADVANCE25 | Yes (computer generated) | Yes (external personnel) | Yes (patients, caring physicians, and outcome assessors) | Yes | No | Yes | Yes | Yes | Low |
| Danzi et al.37 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| ELISA 229 | Unclear | Unclear | Yes (outcome assessors) | No | Yes | Yes | Yes | Yes | Moderate |
| Ercan et al.22 | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | Moderate |
| Ernst et al.42 | Yes (computer generated) | Unclear | No | Yes | No | Yes | Yes | Yes | Moderate |
| EVEREST36 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| FATA38 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Fu et al.30 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Ivandic et al.31 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Juergens et al.18 | Unclear | Unclear | Yes (patients and caring physicians) | No | Yes | Yes | Yes | Yes | Moderate |
| Kereiakes et al.15 | Unclear | Unclear | Yes (patients and caring physicians) | Yes | No | No | No | Yes | Moderate |
| Kim et al.26 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Kurowski et al.27 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| MULTISTRATEGY10 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| NAPLES34 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Neumann et al.40 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Okmen et al.21 | Unclear | Unclear | No | Unclear | No | No | Yes | Yes | Moderate |
| Okmen et al.24 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| On-TIME 2 open-label study33 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| On-TIME 233 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Ozkan et al.28 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| PRISM8 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| PRISM-PLUS7 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| RESTORE6 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| SASTRE23 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| Shen et al.32 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| STRATEGY41 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Moderate |
| TARGET9 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TENACITY43 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TETAMI19,20 | Unclear | Unclear | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TOPSTAR17 | Unclear | Yes (external personnel) | Yes (patients and caring physicians) | Yes | Yes | No | No | Yes | Moderate |
| Study . | Adequate sequence generation . | Allocation concealment used . | Blinding . | Concurrent therapies similar . | Incomplete outcome data addressed . | Uniform and explicit outcome definitions . | Free of selective outcome reporting . | Free of other bias . | Overall risk of bias . |
|---|---|---|---|---|---|---|---|---|---|
| 3T/2R35 | Yes (computer generated) | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| ADVANCE25 | Yes (computer generated) | Yes (external personnel) | Yes (patients, caring physicians, and outcome assessors) | Yes | No | Yes | Yes | Yes | Low |
| Danzi et al.37 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| ELISA 229 | Unclear | Unclear | Yes (outcome assessors) | No | Yes | Yes | Yes | Yes | Moderate |
| Ercan et al.22 | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | Moderate |
| Ernst et al.42 | Yes (computer generated) | Unclear | No | Yes | No | Yes | Yes | Yes | Moderate |
| EVEREST36 | Unclear | Unclear | No | Yes | Yes | No | No | Yes | Moderate |
| FATA38 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Fu et al.30 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Ivandic et al.31 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Juergens et al.18 | Unclear | Unclear | Yes (patients and caring physicians) | No | Yes | Yes | Yes | Yes | Moderate |
| Kereiakes et al.15 | Unclear | Unclear | Yes (patients and caring physicians) | Yes | No | No | No | Yes | Moderate |
| Kim et al.26 | Unclear | Unclear | No | Yes | No | No | Yes | Yes | Moderate |
| Kurowski et al.27 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| MULTISTRATEGY10 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| NAPLES34 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Neumann et al.40 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| Okmen et al.21 | Unclear | Unclear | No | Unclear | No | No | Yes | Yes | Moderate |
| Okmen et al.24 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| On-TIME 2 open-label study33 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| On-TIME 233 | Yes (computer generated) | Yes (centralized system) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| Ozkan et al.28 | Unclear | Unclear | No | Yes | No | No | No | Yes | Moderate |
| PRISM8 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| PRISM-PLUS7 | Unclear | Yes (sealed envelopes) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| RESTORE6 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| SASTRE23 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| Shen et al.32 | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate |
| STRATEGY41 | Yes (computer generated) | Yes (sealed envelopes) | Yes (outcome assessors) | Yes | Yes | Yes | Yes | Yes | Moderate |
| TARGET9 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TENACITY43 | Unclear | Yes (centralized system) | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TETAMI19,20 | Unclear | Unclear | Yes (patients, caring physicians, and outcome assessors) | Yes | Yes | Yes | Yes | Yes | Low |
| TOPSTAR17 | Unclear | Yes (external personnel) | Yes (patients and caring physicians) | Yes | Yes | No | No | Yes | Moderate |
Quantitative synthesis
Tirofiban vs. placebo or bivalirudin
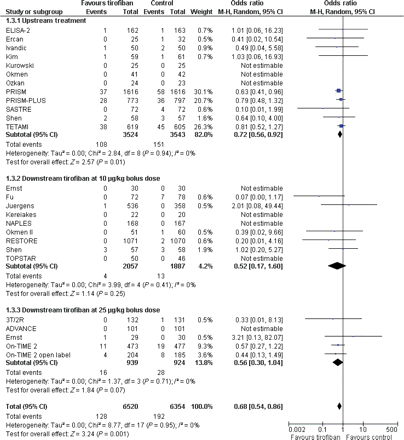
Overall pooled effect estimate analysis showed a significant reduction in short-term (30 days) mortality [OR = 0.68 (0.54–0.86), P = 0.001, P for heterogeneity = 0.95, I2= 0%] (Figure 2), mortality or MI [OR = 0.69 (0.58–0.81), P < 0.001, P for heterogeneity=0.33, I2 = 10%] (Figure 3), MI alone [OR = 0.71 (0.56–0.90), P = 0.004, P for heterogeneity=0.16, I2 = 24%], and the composite of death, MI, or target vessel revascularization [OR = 0.73 (0.60–0.89), P = 0.002, P for heterogeneity = 0.06, I2 = 37%] in patients randomly allocated to receive tirofiban at the different bolus and infusion regimens. According to an absolute risk reduction of 2.5%, the NNT is 40 to prevent one death or MI at 30 days, with an NNT of 100 to prevent one death. Comprehensive heterogeneity and inconsistency analyses showed that included trials led to apparently statistically heterogeneous results in terms of MACE rates (P for heterogeneity = 0.06, I2 = 37%), which is paralleled by the disparities in clinical setting, tirofiban bolus and infusion regimens, interventions, duration of treatment, and outcomes. However, there was no signal of heterogeneity across trials for the composite of death or MI, mortality, or MI alone.
Forest plot of comparison: tirofiban vs. placebo or standard therapy, outcome: 30-day mortality rate. CI, confidence interval; Weight, statistical weight (an indirect estimate of study precision and impact on overall pooled estimates of the single study result).
Forest plot of comparison: tirofiban vs. placebo or standard therapy, outcome: 30-day death or myocardial infarction. CI, confidence interval; Weight, statistical weight (an indirect estimate of study precision and impact on overall pooled estimates of the single study result).
To assess the effect of tirofiban when added to P2Y12 receptor blockers (i.e. ticlopidine or clopidogrel), studies where patients were adequately pretreated with clopidogrel (n = 13) or ticlopidine (n = 1) were selected, comprising 3424 patients.17,22,24,25,27–35,42 Consistent to previous analysis, tirofiban was associated with a significant decrease in mortality [OR = 0.56 (0.34, 0.93), P = 0.02, P for heterogeneity = 0.93, I2 = 0%] and the composite of death or MI [OR = 0.61 (0.48, 0.79), P < 0.001, P for heterogeneity = 0.62, I2 = 0%] at 30 days.
The use of tirofiban tended to increase the rate of major bleeding [1.5 vs. 1.8%; OR = 1.21 (0.88, 1.67), P = 0.24, P for heterogeneity = 0.97, I2 = 0%] with an estimated NNH of 286 for one major haemorrhagic event. Minor bleedings [OR = 1.42 (1.13, 1.79), P = 0.002, P for heterogeneity = 0.97, I2 = 0%, 3.8 vs. 2.7%; NNH: 91] and the incidence of any thrombocytopenia [OR = 1.51 (1.06, 2.16); P = 0.02, P for heterogeneity = 0.96, I2 = 0%] were both significantly increased by the use of tirofiban.
After an average of 5-month follow-up, tirofiban remained associated with a significant reduction in mortality [OR = 0.81 (0.66, 0.99), P = 0.04, P = 0.70 for heterogeneity, I2 = 0%] and death or MI [OR = 0.73 (0.62, 0.85), P < 0.001, P = 0.24 for heterogeneity, I2 = 16%].
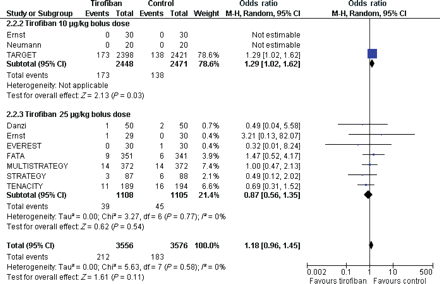
Tirofiban vs. abciximab
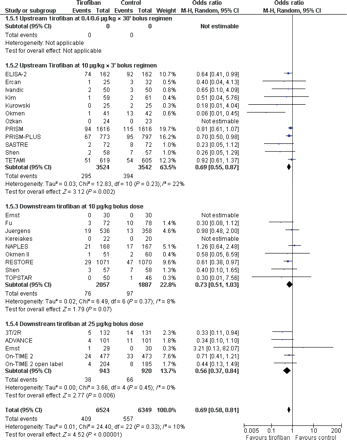
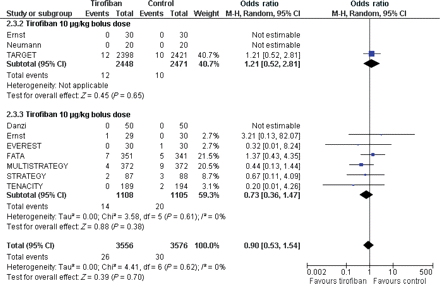
The overall pooled effect estimate analysis showed that tirofiban at 30 days led to similar mortality rate [OR = 0.90 (0.53, 1.54), P = 0.70, P for heterogeneity = 0.62, I2 = 0%] (Figure 4) but tended to increase the composite of death or MI [6.0 vs. 5.1%; OR = 1.18 (0.96, 1.45), P = 0.11, P for heterogeneity = 0.58, I2 = 0%] (Figure 5) and MACE rate [6.3 vs. 5.5%; OR = 1.18 (0.97, 1.44), P = 0.10, P for heterogeneity = 0.43, I2 = 0%] when compared with abciximab. Although there was no formal signal of heterogeneity across included studies, likely due to limited statistical power, these results mainly mirrored the findings of the TARGET study (study weight 78.4%) which tested tirofiban at 10 µg/kg 3 min bolus regimen and 0.15 µg/kg/min infusion. Indeed, mortality [OR = 0.73 (0.36, 1.47), P = 0.38, P for heterogeneity = 0.61, I2 = 0%], the composite of death or MI [OR = 0.87 (0.56, 1.35), P = 0.54, P for heterogeneity = 0.58, I2 = 0%] or MACE rate [OR = 0.87 (0.57, 1.32), P = 0.51, P for heterogeneity = 0.63, I2 = 0%] were similar when tirofiban at high-dose bolus was compared with abciximab.
Forest plot of comparison: tirofiban vs. abciximab, outcome: 30-day death. CI, confidence interval; Weight, statistical weight (an indirect estimate of study precision and impact on overall pooled estimates of the single study result).
Forest plot of comparison: tirofiban vs. abciximab, outcome: 30-day death or myocardial infarction. CI, confidence interval; Weight, statistical weight (an indirect estimate of study precision and impact on overall pooled estimates of the single study result).
The rate of major bleedings did not differ in tirofiban- vs. abciximab-treated patients [OR = 1.24 (0.78, 1.98), P = 0.35, P for heterogeneity = 0.76, I2 = 0%], whereas minor bleedings [3.1 vs. 4.8%; OR = 0.64 (0.50, 0.82), P < 0.001, P for heterogeneity = 0.95, I2 = 0%] and any thrombocytopenia [0.3 vs. 2.4%; OR = 0.28 (0.08, 0.94), P = 0.04, P for heterogeneity=0.71, I2 = 0%] were both markedly reduced in the tirofiban group.
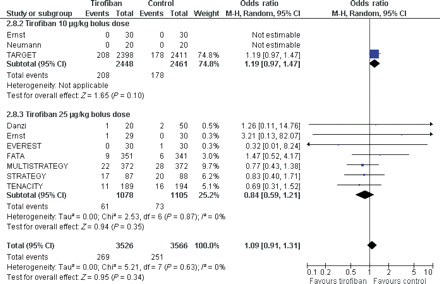
At the longest available follow-up, death or MI [OR = 1.09 (0.91, 1.31), P = 0.34, P for heterogeneity = 0.87, I2 = 0%], mortality [OR = 1.03 (0.75, 1.42), P = 0.86, P for heterogeneity = 0.71, I2 = 0%], and MACE [OR = 1.00 (0.87, 1.16), P = 0.95, P for heterogeneity = 0.68, I2 = 0%] rates also did not differ between groups (Figure 6).
Forest plot of comparison: tirofiban vs. abciximab, outcome: long-term death or myocardial infarction. CI, confidence interval. Weight, statistical weight (an indirect estimate of study precision and impact on overall pooled estimates of the single study result).
Additional analyses
Meta-regression was performed to explore moderators of effect estimates for tirofiban vs. abciximab analysis, focusing on the 30 days rate of death or MI, and on the long-term rate of MACE, and appraising type of control, type of administration, concomitant medical treatment, adequate randomization method, adequate concealment of allocation, and adequate patient blinding (Table 4). The only potentially relevant finding beyond type of control treatment (i.e. placebo or anticoagulant vs. abciximab) was a trend for interaction between 30 days death or MI rates and the comparison between tirofiban and abciximab when focusing on the bolus regimen of tirofiban [β = −0.646 (−1.244, 0.103), P = 0.083], suggestive of more efficacy for the latter when employed at a high (25 µg/kg) dose.
Results of meta-regression analysis
| Variable . | 30 Days death or myocardial infarction . | Long-term major adverse cardiac events . | ||
|---|---|---|---|---|
| Beta (95% confidence interval) . | P-value . | Beta (95% confidence interval) . | P-value . | |
| Type of control | 0.469 (0.127; 0.767) | 0.008 | 0.350 (−0.005; 0.574) | 0.054 |
| Type of administration | 0.056 (−0.136; 0.183) | 0.765 | 0.035 (−0.123; 0.149) | 0.850 |
| Concomitant medical treatment | 0.166 (−0.190; 0.494) | 0.371 | −0.052 (−0.336; 0.255) | 0.782 |
| Adequate randomization method | −0.220 (−0.887; 0.226) | 0.234 | −0.203 (−0.639; 0.187) | 0.273 |
| Adequate concealment of allocation | 0.261 (−0.099; 0.587) | 0.157 | 0.035 (−0.280; 0.337) | 0.850 |
| Adequate patient blinding | 0.362 (0.007; 0.654) | 0.046 | 0.197 (−0.156; 0.507) | 0.289 |
| Variable . | 30 Days death or myocardial infarction . | Long-term major adverse cardiac events . | ||
|---|---|---|---|---|
| Beta (95% confidence interval) . | P-value . | Beta (95% confidence interval) . | P-value . | |
| Type of control | 0.469 (0.127; 0.767) | 0.008 | 0.350 (−0.005; 0.574) | 0.054 |
| Type of administration | 0.056 (−0.136; 0.183) | 0.765 | 0.035 (−0.123; 0.149) | 0.850 |
| Concomitant medical treatment | 0.166 (−0.190; 0.494) | 0.371 | −0.052 (−0.336; 0.255) | 0.782 |
| Adequate randomization method | −0.220 (−0.887; 0.226) | 0.234 | −0.203 (−0.639; 0.187) | 0.273 |
| Adequate concealment of allocation | 0.261 (−0.099; 0.587) | 0.157 | 0.035 (−0.280; 0.337) | 0.850 |
| Adequate patient blinding | 0.362 (0.007; 0.654) | 0.046 | 0.197 (−0.156; 0.507) | 0.289 |
Based on a univariate fixed-effect model with least-squares weights for sample size to explore moderators and/or predictors of changes in log-transformed odds ratios.
Results of meta-regression analysis
| Variable . | 30 Days death or myocardial infarction . | Long-term major adverse cardiac events . | ||
|---|---|---|---|---|
| Beta (95% confidence interval) . | P-value . | Beta (95% confidence interval) . | P-value . | |
| Type of control | 0.469 (0.127; 0.767) | 0.008 | 0.350 (−0.005; 0.574) | 0.054 |
| Type of administration | 0.056 (−0.136; 0.183) | 0.765 | 0.035 (−0.123; 0.149) | 0.850 |
| Concomitant medical treatment | 0.166 (−0.190; 0.494) | 0.371 | −0.052 (−0.336; 0.255) | 0.782 |
| Adequate randomization method | −0.220 (−0.887; 0.226) | 0.234 | −0.203 (−0.639; 0.187) | 0.273 |
| Adequate concealment of allocation | 0.261 (−0.099; 0.587) | 0.157 | 0.035 (−0.280; 0.337) | 0.850 |
| Adequate patient blinding | 0.362 (0.007; 0.654) | 0.046 | 0.197 (−0.156; 0.507) | 0.289 |
| Variable . | 30 Days death or myocardial infarction . | Long-term major adverse cardiac events . | ||
|---|---|---|---|---|
| Beta (95% confidence interval) . | P-value . | Beta (95% confidence interval) . | P-value . | |
| Type of control | 0.469 (0.127; 0.767) | 0.008 | 0.350 (−0.005; 0.574) | 0.054 |
| Type of administration | 0.056 (−0.136; 0.183) | 0.765 | 0.035 (−0.123; 0.149) | 0.850 |
| Concomitant medical treatment | 0.166 (−0.190; 0.494) | 0.371 | −0.052 (−0.336; 0.255) | 0.782 |
| Adequate randomization method | −0.220 (−0.887; 0.226) | 0.234 | −0.203 (−0.639; 0.187) | 0.273 |
| Adequate concealment of allocation | 0.261 (−0.099; 0.587) | 0.157 | 0.035 (−0.280; 0.337) | 0.850 |
| Adequate patient blinding | 0.362 (0.007; 0.654) | 0.046 | 0.197 (−0.156; 0.507) | 0.289 |
Based on a univariate fixed-effect model with least-squares weights for sample size to explore moderators and/or predictors of changes in log-transformed odds ratios.
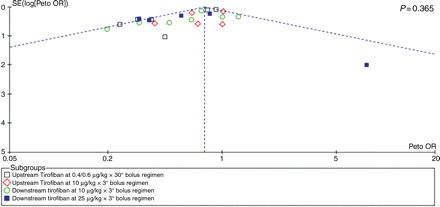
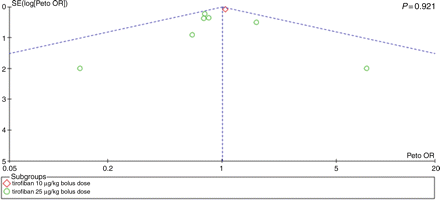
Inspection of funnel plots for either tirofiban vs. control and tirofiban vs. abciximab and the 30 days rate of death or MI (Figures 7 and 8) did not disclose evidence of small study bias, which was also confirmed by analytical testing with Peters test (P = 0.365 and P = 0.921, respectively).
Funnel plot for the long-term risk of major adverse cardiac events (MACE) comparing tirofiban vs. control. This plot shows the association (or lack of) between study effect (x-axis) and study size/precision (y-axis), and can thus provide a graphical appraisal of the risk of small study bias in the overall systematic review. Specifically, small study bias, also known as publication bias, is due to the selective reporting and publication of small but significant studies and the selective under-reporting and lack of publication of small non-significant studies. If present, small study bias may unduly impact on pooled effect estimates and bias the overall results toward rejecting a null hypothesis which is actually valid. The vertical dashed line represents the summary pooled effect estimate, the oblique dashed lines represent the corresponding 95% confidence intervals, and the P-value provided by analytical testing with Peters test. OR, odds ratio; SE, standard error.
Funnel plot for the long-term risk of major adverse cardiac events (MACE) comparing tirofiban vs. abciximab. This plot shows the association (or lack of) between study effect (x-axis) and study size/precision (y-axis), and can thus provide a graphical appraisal of the risk of small study bias in the overall systematic review. Specifically, small study bias, also known as publication bias, is due to the selective reporting and publication of small but significant studies and the selective under-reporting and lack of publication of small non-significant studies. If present, small study bias may unduly impact on pooled effect estimates and bias the overall results toward rejecting a null hypothesis which is actually valid. The vertical dashed line represents the summary pooled effect estimate, the oblique dashed lines represent the corresponding 95% confidence intervals, and the P-value provided by analytical testing with Peters test. OR, odds ratio; SE, standard error.
Discussion
The main finding of this meta-analysis is that adjunctive tirofiban therapy, compared with placebo, is associated with a >30% reduction in all considered ischaemic endpoints including overall mortality, mortality or MI, and MACE rates within 30 days after treatment. In absolute terms, tirofiban administration in 40 patients would prevent one death or MI, whereas 100 treated patients would lead to one fatal event prevention. Importantly, the benefit observed soon after intervention persisted at longest available follow-up. Interestingly, the magnitude of benefit for mortality observed in our analysis for tirofiban was quite similar to the treatment benefit shown by abciximab in a recent meta-analysis.44
As expected, the advantage in terms of ischaemic endpoints was counterbalanced by a significant increase in minor, but not major, bleeding and thrombocytopenia. Assuming that the observed insignificant 25% relative increase in major bleeding in the tirofiban group is real, we estimated a NNH of 286, 91, and 227 to lead to one major bleed, one minor haemorrhagic event, and one episode of thrombocytopenia, respectively. Thus, altogether the use of tirofiban at different tested regimens was associated with a favourable efficacy/safety profile in a broad patient population presenting with acute coronary syndromes and/or undergoing PCI.
While several included studies antedated the advent of clopidogrel pre-treatment strategy in patients undergoing PCI, our sensitivity analysis, which focused on patients receiving tirofiban on top of pretreatment with clopidogrel or ticlopidine,17,22,24,25,27–35,42 suggested the benefit of tirofiban to be additive to first or second generation P2Y12 receptor inhibitors. These findings are in keeping with previous evidence45,46 and reinforce the importance of the degree and consistency of platelet inhibition to prevent ischaemic complications in patients with acute coronary syndromes undergoing PCI.
Our analysis failed to show heterogeneity of results across the different tested regimens of tirofiban for mortality or the composite of death or MI. However, for both MI rate alone and the composite of MACE rate, some degree of inconsistency was noted throughout. This might be due to various MI definitions, multiple clinical settings and/or different tirofiban tested regimens throughout studies. Interestingly, trials testing tirofiban downstream at high bolus dose, which results in a prompt and significantly greater inhibition of platelet activity compared with both standard 10 µg/kg 3 min and 0.4 µg/kg 30 min bolus regimens,3,47,48 resulted in overall numerically higher relative and absolute reduction of death or MI, MI alone, and MACE rates within the first 30 days.
In aggregate, ischaemic complications did not significantly differ in tirofiban vs. abciximab-treated patients at short- or medium-term follow-up. However, tirofiban tested at 10 µg/kg bolus regimen, which results in suboptimal platelet inhibition soon after administration,3,47 increased peri-procedural ischaemic events mainly in terms of MI, compared with abciximab. This was largely driven by the results of the TARGET study, which remains by far the biggest comparison between the two drugs.9 In contrast, the 25 µg/kg tirofiban bolus regimen which has been developed to more closely mimic abciximab-driven platelet inhibition soon after treatment administration,48 was not associated with an increase of early ischaemic hazard when contrasted to the latter. Indeed, a trend was noted suggesting an interaction between 30-day death or MI rates and the comparison between tirofiban and abciximab when focusing on dosage of tirofiban administration. While abciximab treatment effect vs. placebo was previously shown to be directly proportional to risk status of treated patients,49 no such pattern was observed when abciximab was compared with tirofiban at metaregression analysis, suggesting that tirofiban may effectively replace abciximab across the whole spectrum of patients with CAD, particularly with a high-dose bolus regimen.
Altogether, the pooled findings from both placebo and abciximab controlled studies suggest that the bolus regimen, especially for patients undergoing PCI and receiving treatment immediately before is of utmost importance to optimize outcomes. Tirofiban, given at a high-dose bolus, by providing a greater and more consistent level of platelet inhibition may be a preferable option than previously developed standard regimens which lead to desirable anti-platelet activity only with some delay after drug administration.47
Importantly, confidence intervals around point of estimate for ischaemic events remains wide for the comparison between tirofiban and abciximab and entail the possibility that even at high bolus regimen, the former may lead to a relatively small yet distinct increase in adverse events after PCI. This uncertainty largely reflects the still limited number of patients who have been re-evaluated in head-to-head studies with tirofiban given at high bolus dose. Unfortunately, the planned large (n = 8800 patients) TENACITY study which aimed to definitively ascertain whether at proper dosing tirofiban would be non-inferior to abciximab was prematurely stopped for financial reasons after 383 patients were enrolled.43 All subsequent investigator-driven head-to-head comparisons between these two agents were based on surrogate endpoints such as ST-segment elevation resolution,10,38,41 myocardial blush,36 left ventricular ejection fraction,37 or platelet inhibition42 which explains the relatively small study populations.
An additional finding of potential clinical relevance was that the rate of minor, but not major, bleeding was significantly reduced by the use of tirofiban compared with abciximab. This was consistently noted in studies testing either 10 or 25 µg/kg tirofiban bolus regimens. Since the degree of platelet inhibition provided by a high-dose tirofiban bolus is not inferior to that of abciximab, and indeed many previous studies have shown that tirofiban at this revised bolus regimen might be associated with greater and more consistent anti-platelet activity than abciximab,41,42,50 this observation of lower minor bleeding rate in tirofiban-treated patients is intriguing and deserves further investigation. Similarly, the rate of thrombocytopenia, which like bleeding complications has been shown to independently predict worse outcomes,51,52 was reduced by almost 80% by the use of tirofiban. This likely reflects the lower propensity of tirofiban to elicit an antibody response. Thrombocytopenia has been shown to be associated with bleeding complications,51,52 and it is tempting to speculate that the lower propensity of tirofiban to trigger an immune response might at least partially explain the improved safety profile in terms of minor bleedings observed in the tirofiban group. Finally, we cannot rule out the possibility that the difference in minor bleedings noted between tirofiban and abciximab is a spurious finding or simply related to the shorter duration of anti-aggregatory effect.
Study limitations
Our results suffer from those limitations which are inherent to all meta-analytic techniques including particularly heterogeneity in patient populations, different study drug regimens, and variable endpoint definitions across studies. This mainly applies to the different criteria employed throughout trials for classifying bleeding and peri-procedural ischaemic endpoints. Importantly, however, a clear reduction of overall mortality in the tirofiban arm has been noted vs. placebo but not vs. abciximab studies which is in keeping with the differences observed between study groups for MI alone or the composite of death or MI.
Conclusions
In our pooled analysis based on over 20 000 patients, tirofiban administration was shown to significantly reduce mortality, the composite of death or MI along with MACE rate when compared with placebo. This benefit in ischaemic endpoints reduction remained significant and of consistent magnitude in studies where tirofiban was tested in addition to thienopyridines but came at an increase risk for minor bleeding and thrombocytopenia. An early ischaemic hazard disfavouring tirofiban was noted when compared with abciximab in studies based on 10 µg/kg bolus regimen but not in those testing the 25 µg/kg bolus regimen. Overall, the safety profile seems to favour the use of tirofiban over abciximab for lower incidence of minor bleeding and thrombocytopenia, likely reflecting different chemical structures more than a difference in anti-platelet potency between these two drugs.
Our findings suggest that the use of tirofiban is an efficacious treatment option to reduce ischaemic events in patients with acute coronary syndromes and/or those undergoing PCI. When employed at high-dose bolus just prior to PCI, tirofiban may provide similar efficacy yet an improved safety profile when compared with abciximab. This hypothesis would require prospective assessment in order to be validated.
Funding
This work was supported by the University of Ferrara, Italy.
Conflicts of interest: M.V. has consulted for Iroko, Eli Lilly and the Medicines Company, has lectured for Iroko, Glaxo SmithKline and received grant support from Iroko and Eli Lilly. G.B.-Z. has consulted for Cordis and The Medicines Company, has lectured for Bristol-Myers Squibb and sanofi-aventis, and has received grant support from Glaxo SmithKline.