-
PDF
- Split View
-
Views
-
Cite
Cite
Artur Fedorowski, Lars Stavenow, Bo Hedblad, Göran Berglund, Peter M. Nilsson, Olle Melander, Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmö Preventive Project), European Heart Journal, Volume 31, Issue 1, January 2010, Pages 85–91, https://doi.org/10.1093/eurheartj/ehp329
Close - Share Icon Share
Abstract
Orthostatic hypotension (OH) has been linked to increased mortality and incidence of cardiovascular disease in various risk groups, but determinants and consequences of OH in the general population are poorly studied.
Prospective data of the Swedish ‘Malmö Preventive Project’ (n = 33 346, 67.3% men, mean age 45.7 ± 7.4 years, mean follow-up 22.7 ± 6.0 years) were analysed. Orthostatic hypotension was found in 6.2% of study participants and was associated with age, female gender, hypertension, antihypertensive treatment, increased heart rate, diabetes, low BMI, and current smoking. In Cox regression analysis, individuals with OH had significantly increased all-cause mortality (in particular those aged less than 42 years) and coronary event (CE) risk. Mortality and CE risk were distinctly higher in those with systolic blood pressure (BP) fall ≥30 mmHg [hazard ratio (HR): 1.6, 95% CI 1.3–1.9, P < 0.0001 and 1.6, 95% CI 1.2–2.1, P = 0.001] and diastolic BP fall ≥15 mmHg (HR: 1.4, 95% CI 1.1–1.9, P = 0.024 and 1.7, 95% CI 1.1–2.5, P = 0.01). In addition, impaired diastolic BP response had relatively greater impact (per mmHg) on CE incidence than systolic reaction.
Orthostatic hypotension can be detected in ∼6% of middle-aged individuals and is often associated with such comorbidities as hypertension or diabetes. Presence of OH increases mortality and CE risk, independently of traditional risk factors. Although both impaired systolic and diastolic responses predict adverse events, the diastolic impairment shows stronger association with coronary disease.
Introduction
Orthostatic blood pressure (BP) control involves complex compensatory mechanisms allowing the human body to stand upright.1 As the postural homeostasis is principally mediated by autonomic nervous system, its impairment may lead to BP fall after standing. The phenomenon, denominated as orthostatic hypotension (OH), is often associated with debilitating symptoms: fatigue, dizziness, and fainting.2–4 Orthostatic hypotension has been defined by the international consensus as a decrease in systolic BP ≥ 20 mmHg and/or decrease in diastolic BP ≥ 10 mmHg within 3 min of standing.5,6 In addition, some authors have proposed standing systolic BP < 90 mmHg as an alternative criterion.7
Clinicians are usually interested in diagnosing OH as it can cause fall-related injuries,8 substantially limit patients' quality of life,9 and finally, impede relevant treatment of concomitant diseases as hypertension or heart failure.10,11 In parallel, relatively little is known about prognostic aspects of OH. Increased mortality and incidence of cardiovascular disease (CVD) related to prevalent OH has been reported in different high-risk groups12–14 with dominantly symptomatic patients. However, the number of studies regarding the prognostic value of younger and mainly asymptomatic individuals without significant burden of co-morbidities is limited. Prospective data from the Atherosclerosis Risk in Communities (ARIC) study suggest that OH may confer higher risk of all-cause mortality and cardiovascular events.15–17
Consequently, the aim of our study was to explore prevalence, determinants, and major consequences [total mortality, coronary event (CE), and stroke] of impaired postural haemodynamic response in a Swedish urban middle-aged cohort of the Malmö Preventive Project (MPP).
Methods
Study population and baseline examination
The Malmö Preventive Project was started in the mid-1970s at the Malmö University Hospital,18 which is the main hospital in the Malmö city area (population of ∼280 000). Between 1974 and 1992, a total of 22 444 men and 10 902 women were recruited and screened for hypertension, diabetes, hyperlipidaemia, smoking, family history, previous diseases, and other all-cause mortality and CVD risk factors. Specially trained nurses performed all examinations in the morning. The invited men and women were fasting overnight prior to investigation. Height and weight were measured in light indoor clothing. A complete medication list was recorded for each participant. Blood samples were collected and plasma lipids (total cholesterol and triglycerides) were analysed by routine methods at the Department of Clinical Chemistry, Malmö University Hospital. Fasting plasma glucose was analysed with a hexokinase method. Afterwards, the participants answered a questionnaire of ∼260 questions using a computer. The questions centred on own and family history of CVD (myocardial infarction and stroke), hypertension, diabetes and cancer, smoking habits (those who confirmed regular or occasional current smoking were counted as smokers), physical activity at work and during leisure time, dietary habits and weight gain, alcohol consumption, presence of symptoms and signs indicating coronary heart disease, cardiac failure, hypertension, or stroke. The following questions were valid for the history of CVD, if the answer was yes: have you ever been hospitalized for myocardial infarction/stroke? Men were recruited mainly in the years 1974–1981 and woman in the years 1982–1992, which resulted in different mean age in these two groups at the screening [men (mean age ± SD; range, in years): 43.7 ± 6.6; 26.5–61.2 and women: 49.7 ± 7.4; 28.2–57.6]. A detailed description of recruitment and screening procedures may be found elsewhere.18–20
Blood pressure measurements
Blood pressure (mmHg) was measured auscultatorily by specially trained nurses in two different positions (supine and standing), with a mercury sphygmomanometer and an appropriate cuff placed around the right arm. First BP reading was taken after 10 min rest in the supine position. Then the participants were asked to stand up and the second BP measurement was taken after 1 min. Blood pressure was determined and recorded to the nearest 5 mmHg. Complete data on 32 797 participants were available (missing data n = 549).
Definition of orthostatic hypotension
Orthostatic hypotension at the baseline was defined according to the international consensus as a decrease in systolic BP ≥ 20 mmHg and/or decrease in diastolic BP ≥10 mmHg within 3 min of standing, or, in addition, as standing systolic BP <90 mmHg.
Definition of hypertension and diabetes
For the purposes of this study, hypertension at the screening was defined according to the present guidelines as systolic BP ≥ 140 mmHg and/or diastolic BP ≥90 mmHg, or use of antihypertensive treatment.21
Diabetes was defined as fasting plasma glucose ≥7.0 mmol/L or current pharmacological treatment of diabetes or self-reported history of diabetes.
Follow-up and endpoints
All participants were followed-up until the 31 December 2005 through linkage of the 10-digit personal identification number of each Swedish citizen with three registries: the Swedish National Hospital Discharge Register (SNHDR), the Swedish National Cause of Death Register (SNCDR), and the Stroke Register of Malmö (STROMA). Participants who emigrated from Sweden before 31 December 2005 and had been event-free at the time of emigration (n = 641) received the date of emigration as the last follow-up date. The mean follow-up time was 22.7 ± 6.0 years.
Coronary event was defined as fatal or non-fatal myocardial infarction or death due to coronary heart disease on the basis of the International Classification of Diseases 9th and 10th Revisions (ICD9 and ICD10) codes 410 and I21, respectively, in the SNHDR and codes 410, 412, and 414 (ICD9) or I21–I23 and I25 (ICD10) in the SNCDR. The register-based diagnosis of CE in the SNHDR has been found to be highly valid.22
Fatal or non-fatal stroke was defined according to ICD9 and ICD10 as cases coded 430, 431, 434, and 436 or I60, I61, I63, and I64, respectively. STROMA, which continuously and actively has searched for and validated patients with stroke since 1989 (including imaging techniques),23 was used for case retrieval. The research nurse, together with a senior physician, validated the diagnosis through medical records and in most cases also through a patient interview. The criterion for stroke was rapid development of clinical signs of local or global loss of cerebral function lasting for >24 h or leading to death at <24 h, with no apparent cause other than cerebral ischaemia or haemorrhage. By definition, patients with transient ischaemic attacks were excluded. The criterion for stroke classified as subarachnoid haemorrhage or intracerebral haemorrhage was verification of the clinical picture by CT, lumbar puncture, or necropsy.24 For those participants who experienced stroke before 1989, available medical records were validated using the same procedure as STROMA. In addition, SNHDR and SNCDR were used for retrieval of patients who moved out of Malmö. In subjects with more than one CE or stroke, only the first event was used for the analysis.
Statistical analysis
Groupwise differences in continuous variables were compared using t-test and variables that were not normally distributed were log-transformed prior to analysis. Dichotomous variables were compared using χ2 test. Presence of OH at the baseline was first related to different covariates in an unadjusted model. Thereafter, we performed a logistic regression analysis, entering OH as the dependent variable and age, gender, body mass index (BMI), total cholesterol, triglycerides, heart rate, haemoglobin, creatinine, hypertension, antihypertensive treatment, diabetes, current smoking, history of CVD, and history of cancer as independent variables, in order to identify determinants of OH.
Presence of OH at the baseline was related to all-cause mortality, first-incident CE, and first-incident stroke and, additionally, to first composite endpoint (first-incident CV event, stroke or CE, or death) during follow-up in crude and multivariate adjusted Cox proportional hazard models. All participants with positive history of CVD prior to the baseline examination (n = 159) were excluded. The multivariate adjusted analyses included the following covariates: age, gender, current smoking, BMI, hypertension, diabetes, total cholesterol (HDL and LDL cholesterol were not analysed in MPP), and previous cancer (for mortality analysis only).
Analyses were repeated for three different age groups: less than 42 years (n = 9395), 42–48 years (n = 11 043), and ≥48 years (n = 12 357) as previous reports suggested that younger OH positive individuals might be at relatively higher mortality risk.16
Furthermore, analyses were also repeated for conventional (systolic BP fall ≥20 mmHg and diastolic BP fall ≥10 mmHg) and higher cut-off limits (systolic BP fall ≥30 mmHg and diastolic BP fall ≥15 mmHg) as BP values recorded at baseline examination were rounded to the nearest 5 mmHg, which might have classified some borderline cases as falsely OH positive. Systolic and diastolic covariates were analysed separately.
In addition, associations between a continuous variable—orthostatic BP reaction (OBPR, Δ mmHg=supine BP−standing BP) and all three endpoints were examined using the same Cox proportional hazard models, separately for systolic and diastolic OBPR. In these analyses, OBPR was adjusted for supine systolic BP and antihypertensive treatment instead of the dichotomous variable of hypertension. Potential interactions between supine systolic BP and OBPR were assessed.
All calculations were performed using SPSS statistical software version 16.0 for Windows (SPSS Inc., 233 S. Wacker Drive, Chicago, IL 60606-6307, USA). All tests were two-sided and a P < 0.05 was considered statistically significant.
Results
In total, 6.2% of study participants (n = 2033) were found having OH at the baseline. Orthostatic hypotension prevalence in men was 5.3% (n = 1186) and in women 8.1% (n = 847).
One thousand three hundred and seventy-four individuals (4.1%) were classified as OH positive according to systolic OBPR ≥ 20 mmHg and 802 (2.4%) based on diastolic OBPR ≥ 10 mmHg. Only 168 individuals (0.5%) fulfilled both criteria. Additionally, 25 participants (0.1%) had standing systolic BP < 90 mmHg.
As can be seen in Table 1, OH positive individuals were older, more likely to be women and current smokers. Furthermore, OH was correlated with statistically significant higher prevalence of hypertension, diabetes, previous CVD, and antihypertensive treatment, as well as with higher heart rate, systolic and diastolic BP, and triglycerides at the baseline screening. In the multivariate analysis, the following independent OH determinants were identified: age, per year [odds ratio (OR): 1.05, 95% confidence interval (CI) 1.04–1.06], female gender (OR: 1.29, 95% CI 1.16–1.43), hypertension (OR: 2.35, 95% CI 2.10–2.62), antihypertensive treatment (OR: 1.35, 95% CI 1.15–1.60), diabetes (OR: 1.38, 95% CI 1.13–1.67), BMI, per kg/m2 (OR: 0.96, 95% CI 0.95–0.97), current smoking (OR: 1.37, 95% CI 1.24–1.52, P < 0.001 for all), and heart rate, per beat/min (OR: 1.006, 95% CI 1.001–1.011, P = 0.013).
Correlates of orthostatic hypotension in the Malmö Preventive Project in an unadjusted model presented as means with standard deviation or percentages
| Covariate . | OH negative (n = 30 764) . | OH positive (n = 2033) . | P-value . |
|---|---|---|---|
| Age (years) | 45.4 ± 7.4 | 48.8 ± 7.2 | <0.001 |
| Gender (male, %) | 68.9 | 58.3 | <0.001 |
| BMI | 24.57 ± 3.6 | 24.62 ± 4.0 | 0.505 |
| Total cholesterol (mmol/L) | 5.7 ± 1.1 | 5.8 ± 1.2 | <0.001 |
| Triglycerides (mmol/L) | 1.38 ± 0.9 | 1.44 ± 1.1 | 0.007 |
| Heart rate (b.p.m.) | 67.4 ± 9.7 | 68.9 ± 10.8 | <0.001 |
| Systolic BP (mmHg) | 125.7 ± 14.9 | 136.3 ± 20.2 | <0.001 |
| Diastolic BP (mmHg) | 84.1 ± 9.5 | 88.1 ± 11.3 | <0.001 |
| Haemoglobin (g/L) | 144.3 ± 12.0 | 143.0 ± 12.6 | <0.001 |
| Creatinine (µmol/L) | 87.8 ± 18.8 | 86.3 ± 17.9 | <0.001 |
| Hypertension (%) | 38.9 | 61.4 | <0.001 |
| Diabetes (%) | 4.6 | 7.4 | <0.001 |
| Current smoker (%) | 45.1 | 48.7 | 0.002 |
| History of CVD (%) | 0.5 | 1.0 | 0.001 |
| History of cancer (%) | 1.7 | 2.1 | 0.215 |
| Antihypertensive treatment (%) | 5.0 | 12.1 | <0.001 |
| Covariate . | OH negative (n = 30 764) . | OH positive (n = 2033) . | P-value . |
|---|---|---|---|
| Age (years) | 45.4 ± 7.4 | 48.8 ± 7.2 | <0.001 |
| Gender (male, %) | 68.9 | 58.3 | <0.001 |
| BMI | 24.57 ± 3.6 | 24.62 ± 4.0 | 0.505 |
| Total cholesterol (mmol/L) | 5.7 ± 1.1 | 5.8 ± 1.2 | <0.001 |
| Triglycerides (mmol/L) | 1.38 ± 0.9 | 1.44 ± 1.1 | 0.007 |
| Heart rate (b.p.m.) | 67.4 ± 9.7 | 68.9 ± 10.8 | <0.001 |
| Systolic BP (mmHg) | 125.7 ± 14.9 | 136.3 ± 20.2 | <0.001 |
| Diastolic BP (mmHg) | 84.1 ± 9.5 | 88.1 ± 11.3 | <0.001 |
| Haemoglobin (g/L) | 144.3 ± 12.0 | 143.0 ± 12.6 | <0.001 |
| Creatinine (µmol/L) | 87.8 ± 18.8 | 86.3 ± 17.9 | <0.001 |
| Hypertension (%) | 38.9 | 61.4 | <0.001 |
| Diabetes (%) | 4.6 | 7.4 | <0.001 |
| Current smoker (%) | 45.1 | 48.7 | 0.002 |
| History of CVD (%) | 0.5 | 1.0 | 0.001 |
| History of cancer (%) | 1.7 | 2.1 | 0.215 |
| Antihypertensive treatment (%) | 5.0 | 12.1 | <0.001 |
Correlates of orthostatic hypotension in the Malmö Preventive Project in an unadjusted model presented as means with standard deviation or percentages
| Covariate . | OH negative (n = 30 764) . | OH positive (n = 2033) . | P-value . |
|---|---|---|---|
| Age (years) | 45.4 ± 7.4 | 48.8 ± 7.2 | <0.001 |
| Gender (male, %) | 68.9 | 58.3 | <0.001 |
| BMI | 24.57 ± 3.6 | 24.62 ± 4.0 | 0.505 |
| Total cholesterol (mmol/L) | 5.7 ± 1.1 | 5.8 ± 1.2 | <0.001 |
| Triglycerides (mmol/L) | 1.38 ± 0.9 | 1.44 ± 1.1 | 0.007 |
| Heart rate (b.p.m.) | 67.4 ± 9.7 | 68.9 ± 10.8 | <0.001 |
| Systolic BP (mmHg) | 125.7 ± 14.9 | 136.3 ± 20.2 | <0.001 |
| Diastolic BP (mmHg) | 84.1 ± 9.5 | 88.1 ± 11.3 | <0.001 |
| Haemoglobin (g/L) | 144.3 ± 12.0 | 143.0 ± 12.6 | <0.001 |
| Creatinine (µmol/L) | 87.8 ± 18.8 | 86.3 ± 17.9 | <0.001 |
| Hypertension (%) | 38.9 | 61.4 | <0.001 |
| Diabetes (%) | 4.6 | 7.4 | <0.001 |
| Current smoker (%) | 45.1 | 48.7 | 0.002 |
| History of CVD (%) | 0.5 | 1.0 | 0.001 |
| History of cancer (%) | 1.7 | 2.1 | 0.215 |
| Antihypertensive treatment (%) | 5.0 | 12.1 | <0.001 |
| Covariate . | OH negative (n = 30 764) . | OH positive (n = 2033) . | P-value . |
|---|---|---|---|
| Age (years) | 45.4 ± 7.4 | 48.8 ± 7.2 | <0.001 |
| Gender (male, %) | 68.9 | 58.3 | <0.001 |
| BMI | 24.57 ± 3.6 | 24.62 ± 4.0 | 0.505 |
| Total cholesterol (mmol/L) | 5.7 ± 1.1 | 5.8 ± 1.2 | <0.001 |
| Triglycerides (mmol/L) | 1.38 ± 0.9 | 1.44 ± 1.1 | 0.007 |
| Heart rate (b.p.m.) | 67.4 ± 9.7 | 68.9 ± 10.8 | <0.001 |
| Systolic BP (mmHg) | 125.7 ± 14.9 | 136.3 ± 20.2 | <0.001 |
| Diastolic BP (mmHg) | 84.1 ± 9.5 | 88.1 ± 11.3 | <0.001 |
| Haemoglobin (g/L) | 144.3 ± 12.0 | 143.0 ± 12.6 | <0.001 |
| Creatinine (µmol/L) | 87.8 ± 18.8 | 86.3 ± 17.9 | <0.001 |
| Hypertension (%) | 38.9 | 61.4 | <0.001 |
| Diabetes (%) | 4.6 | 7.4 | <0.001 |
| Current smoker (%) | 45.1 | 48.7 | 0.002 |
| History of CVD (%) | 0.5 | 1.0 | 0.001 |
| History of cancer (%) | 1.7 | 2.1 | 0.215 |
| Antihypertensive treatment (%) | 5.0 | 12.1 | <0.001 |
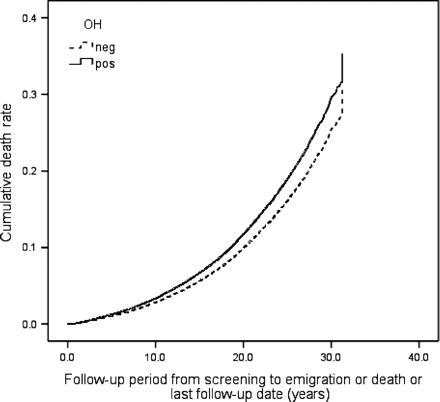
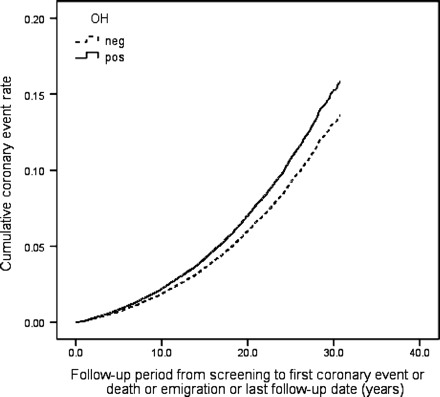
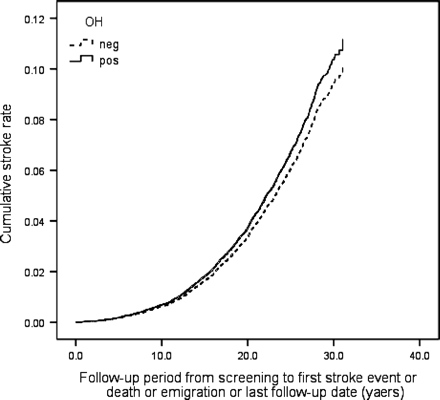
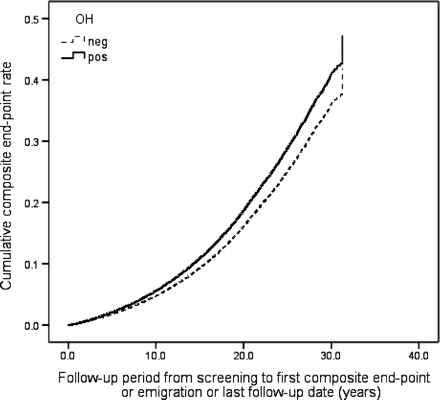
During the follow-up period 6904 individuals (20.7%) died, 3849 (11.5%) suffered from CE and 2134 (6.4%) were diagnosed with incident stroke (86.4% ischaemic, 12.6% haemorrhagic, 1.0% unspecified). Orthostatic hypotension positive individuals demonstrated higher all-cause mortality risk, both in crude [hazard ratio (HR): 1.66, 95% CI 1.53–1.81, P < 0.001] and in adjusted model (HR: 1.19, 95% CI 1.09–1.30, P < 0.001), higher CE risk in crude (HR: 1.58, 95% CI 1.41–1.77, P < 0.001) and adjusted model (HR: 1.18, 95% CI 1.05–1.33, P = 0.007), and higher risk of composite endpoint in both models (HR: 1.59, 95% CI 1.47–1.71, P < 0.001 and 1.18, 1.09–1.27, P < 0.001). Orthostatic hypotension was also related to higher stroke risk in a crude model (HR: 1.59, 95% CI 1.36–1.85, P < 0.001), but this association was attenuated after adjustment for conventional CVD risk factors (HR: 1.11, 95% CI 0.95–1.30, P = 0.21). Effects of baseline OH status on all-cause mortality, incidence of CE, stroke, and composite endpoint in the MPP cohort are presented as multivariate adjusted Cox regression plots in Figures 123–4.
Orthostatic hypotension (OH) and all-cause mortality. One Minus Survival Function adjusted for age, gender, BMI, hypertension, diabetes, total cholesterol, smoking, and previous cancer.
Orthostatic hypotension (OH) and coronary events. One Minus Coronary Event–Free Survival Function adjusted for age, gender, BMI, hypertension, diabetes, total cholesterol, and smoking.
Orthostatic hypotension (OH) and stroke. One Minus Stroke Event–Free Survival Function adjusted for age, gender, BMI, hypertension, diabetes, total cholesterol, and smoking.
Orthostatic hypotension (OH) and composite endpoint (CE, stroke, and death). One Minus Event–Free Survival Function adjusted for age, gender, BMI, hypertension, diabetes, total cholesterol, and smoking.
While repeating analyses in the predefined age groups (Table 2), the following observations were made: mortality risk was more than two times higher in OH positive individuals who were younger than 42 years, and conversely, incidence of CE in the same age group showed no significant association with the baseline OH. In parallel, impact of OH on all-cause mortality and CE in the two remaining age groups (42–48 years and over 48 years) was substantially the same as for the whole cohort, whereas association between baseline OH and composite endpoint was attenuated with increasing age.
Orthostatic hypotension related risk of mortality, coronary event, and stroke by age-stratified subgroups of MPP in multivariatea adjusted Cox regression model (hazard ratio, 95% confidence interval, and P-value)
| Age groups . | <42 years . | ≥42 and <48 years . | ≥48 years . |
|---|---|---|---|
| Total number of individuals | 9396 | 11 044 | 12 357 |
| Male (%) | 83.3 | 88.6 | 37.3 |
| OH positive (%) | 3.1 | 5.7 | 9.0 |
| Number of deaths/CEs/strokes | 920/606/292 | 2545/1559/820 | 3 381/1662/1001 |
| Events (death, CE, and stroke) per 1000 person-years | 3.90/2.57/1.24 | 9.85/6.03/3.17 | 13.41/6.59/3.97 |
| All-cause mortality risk | 1.46 (1.07–2.00), P = 0.017 | 1.21 (1.04–1.41), P = 0.012 | 1.17 (1.04–1.31), P = 0.007; |
| CE risk | 1.06 (0.68–1.67), P = 0.786 | 1.19 (0.98–1.45), P = 0.077 | 1.20 (1.03–1.40), P = 0.022 |
| Stroke risk | 0.92 (0.47–1.79), P = 0.799 | 1.16 (0.88–1.52) P = 0.300 | 1.13 (0.92–1.38) P = 0.251 |
| Composite endpoint (CE, stroke, or death) risk | 1.27 (0.98–1.65) P = 0.073 | 1.24 (1.09–1.41) P = 0.001 | 1.14 (1.03–1.26) P = 0.010 |
| Age groups . | <42 years . | ≥42 and <48 years . | ≥48 years . |
|---|---|---|---|
| Total number of individuals | 9396 | 11 044 | 12 357 |
| Male (%) | 83.3 | 88.6 | 37.3 |
| OH positive (%) | 3.1 | 5.7 | 9.0 |
| Number of deaths/CEs/strokes | 920/606/292 | 2545/1559/820 | 3 381/1662/1001 |
| Events (death, CE, and stroke) per 1000 person-years | 3.90/2.57/1.24 | 9.85/6.03/3.17 | 13.41/6.59/3.97 |
| All-cause mortality risk | 1.46 (1.07–2.00), P = 0.017 | 1.21 (1.04–1.41), P = 0.012 | 1.17 (1.04–1.31), P = 0.007; |
| CE risk | 1.06 (0.68–1.67), P = 0.786 | 1.19 (0.98–1.45), P = 0.077 | 1.20 (1.03–1.40), P = 0.022 |
| Stroke risk | 0.92 (0.47–1.79), P = 0.799 | 1.16 (0.88–1.52) P = 0.300 | 1.13 (0.92–1.38) P = 0.251 |
| Composite endpoint (CE, stroke, or death) risk | 1.27 (0.98–1.65) P = 0.073 | 1.24 (1.09–1.41) P = 0.001 | 1.14 (1.03–1.26) P = 0.010 |
aCox proportional hazard model adjusted for age, gender, hypertension, total cholesterol, diabetes, BMI, current smoking, and cancer (mortality only).
Orthostatic hypotension related risk of mortality, coronary event, and stroke by age-stratified subgroups of MPP in multivariatea adjusted Cox regression model (hazard ratio, 95% confidence interval, and P-value)
| Age groups . | <42 years . | ≥42 and <48 years . | ≥48 years . |
|---|---|---|---|
| Total number of individuals | 9396 | 11 044 | 12 357 |
| Male (%) | 83.3 | 88.6 | 37.3 |
| OH positive (%) | 3.1 | 5.7 | 9.0 |
| Number of deaths/CEs/strokes | 920/606/292 | 2545/1559/820 | 3 381/1662/1001 |
| Events (death, CE, and stroke) per 1000 person-years | 3.90/2.57/1.24 | 9.85/6.03/3.17 | 13.41/6.59/3.97 |
| All-cause mortality risk | 1.46 (1.07–2.00), P = 0.017 | 1.21 (1.04–1.41), P = 0.012 | 1.17 (1.04–1.31), P = 0.007; |
| CE risk | 1.06 (0.68–1.67), P = 0.786 | 1.19 (0.98–1.45), P = 0.077 | 1.20 (1.03–1.40), P = 0.022 |
| Stroke risk | 0.92 (0.47–1.79), P = 0.799 | 1.16 (0.88–1.52) P = 0.300 | 1.13 (0.92–1.38) P = 0.251 |
| Composite endpoint (CE, stroke, or death) risk | 1.27 (0.98–1.65) P = 0.073 | 1.24 (1.09–1.41) P = 0.001 | 1.14 (1.03–1.26) P = 0.010 |
| Age groups . | <42 years . | ≥42 and <48 years . | ≥48 years . |
|---|---|---|---|
| Total number of individuals | 9396 | 11 044 | 12 357 |
| Male (%) | 83.3 | 88.6 | 37.3 |
| OH positive (%) | 3.1 | 5.7 | 9.0 |
| Number of deaths/CEs/strokes | 920/606/292 | 2545/1559/820 | 3 381/1662/1001 |
| Events (death, CE, and stroke) per 1000 person-years | 3.90/2.57/1.24 | 9.85/6.03/3.17 | 13.41/6.59/3.97 |
| All-cause mortality risk | 1.46 (1.07–2.00), P = 0.017 | 1.21 (1.04–1.41), P = 0.012 | 1.17 (1.04–1.31), P = 0.007; |
| CE risk | 1.06 (0.68–1.67), P = 0.786 | 1.19 (0.98–1.45), P = 0.077 | 1.20 (1.03–1.40), P = 0.022 |
| Stroke risk | 0.92 (0.47–1.79), P = 0.799 | 1.16 (0.88–1.52) P = 0.300 | 1.13 (0.92–1.38) P = 0.251 |
| Composite endpoint (CE, stroke, or death) risk | 1.27 (0.98–1.65) P = 0.073 | 1.24 (1.09–1.41) P = 0.001 | 1.14 (1.03–1.26) P = 0.010 |
aCox proportional hazard model adjusted for age, gender, hypertension, total cholesterol, diabetes, BMI, current smoking, and cancer (mortality only).
In the multivariate adjusted model, all-cause mortality, CE, and composite endpoint risks were distinctly higher in those participants who fulfilled more restrictive OH criteria: systolic BP fall ≥30 mmHg (n = 220; HR: 1.59, 95% CI 1.29–1.97, P < 0.001; 1.60, 1.21–2.10, P = 0.001; and 1.42, 1.18–1.72, P < 0.001, respectively) or diastolic BP fall ≥15 mmHg (n = 124; HR: 1.40, 95% CI 1.01–1.93, P = 0.042; 1.65, 1.10–2.46, P = 0.015; and 1.35, 1.02–1.77, P = 0.035, respectively). In parallel, conventional diagnostic limits were associated with relative risk corresponding to that of pooled OH group (data not shown).
The mean and standard deviation values of systolic and diastolic OBPR were 1.2 ± 8.8 and −2.2 ± 5.4 mmHg, respectively. As shown in Table 3, both systolic and diastolic OBPR were related to increased mortality, and higher CE and stroke incidence in the crude models. After adjustment for conventional risk factors, although both systolic and diastolic reactions predicted higher all-cause mortality, increased CE risk remained significantly associated with diastolic BP fall only. Only one significant (P = 0.028) interaction was detected between supine systolic BP and systolic OBPR in the analysis of total mortality. We decided therefore to repeat this analysis in the following subgroups stratified by systolic BP <140 mmHg (normotensive), ≥140 and <160 mmHg (mild hypertension), and ≥160 mmHg (moderate-to-severe hypertension). The strongest association between systolic OBPR and all-cause mortality was found in the middle stratum ≥140 and <160 mmHg [HR (per 10 mmHg): 1.07, 95% CI 1.02–1.12, P = 0.011], then in the normotensive individuals (1.04, 1.00–1.08, P = 0.029), whereas association in the highest stratum ≥160 mmHg was not significant (1.03, 0.95–1.11, P = 0.423).
Relationships between orthostatic blood pressure reaction and all-cause mortality, coronary event, stroke, and composite endpoint in crude and adjusted Cox regression models (hazard ratio, 95% confidence interval, P-value)
| Endpoint . | Systolic OBPRa . | Diastolic OBPRa . |
|---|---|---|
| All-cause mortality | 1.21 (1.18–1.23), P < 0.001 | 1.18 (1.13–1.22), P < 0.001 |
| 1.05 (1.02–1.07), P = 0.001b | 1.05 (1.01–1.10), P = 0.017b | |
| Coronary event | 1.17 (1.13–1.21), P < 0.001 | 1.20 (1.14–1.26), P < 0.001 |
| 1.02 (0.99–1.06), P = 0.213b | 1.09 (1.03–1.15), P = 0.003b | |
| Stroke | 1.17 (1.12–1.22), P < 0.001 | 1.19 (1.11–1.27), P < 0.001 |
| 0.98 (0.93–1.03), P = 0.488b | 1.06 (0.99–1.14), P = 0.111b | |
| Composite endpoint (CE, stroke, or death) | 1.18 (1.15–1.20), P < 0.001 | 1.17 (1.13–1.21), P < 0.001 |
| 1.04 (1.02–1.06), P = 0.001b | 1.05 (1.01–1.09), P = 0.011b | |
| Endpoint . | Systolic OBPRa . | Diastolic OBPRa . |
|---|---|---|
| All-cause mortality | 1.21 (1.18–1.23), P < 0.001 | 1.18 (1.13–1.22), P < 0.001 |
| 1.05 (1.02–1.07), P = 0.001b | 1.05 (1.01–1.10), P = 0.017b | |
| Coronary event | 1.17 (1.13–1.21), P < 0.001 | 1.20 (1.14–1.26), P < 0.001 |
| 1.02 (0.99–1.06), P = 0.213b | 1.09 (1.03–1.15), P = 0.003b | |
| Stroke | 1.17 (1.12–1.22), P < 0.001 | 1.19 (1.11–1.27), P < 0.001 |
| 0.98 (0.93–1.03), P = 0.488b | 1.06 (0.99–1.14), P = 0.111b | |
| Composite endpoint (CE, stroke, or death) | 1.18 (1.15–1.20), P < 0.001 | 1.17 (1.13–1.21), P < 0.001 |
| 1.04 (1.02–1.06), P = 0.001b | 1.05 (1.01–1.09), P = 0.011b | |
aOBPR effects are presented for 10 mmHg difference.
bCox proportional hazard model adjusted for age, gender, SBP, AHT, total cholesterol, diabetes, BMI, current smoking, previous CVD, and cancer (mortality only).
Relationships between orthostatic blood pressure reaction and all-cause mortality, coronary event, stroke, and composite endpoint in crude and adjusted Cox regression models (hazard ratio, 95% confidence interval, P-value)
| Endpoint . | Systolic OBPRa . | Diastolic OBPRa . |
|---|---|---|
| All-cause mortality | 1.21 (1.18–1.23), P < 0.001 | 1.18 (1.13–1.22), P < 0.001 |
| 1.05 (1.02–1.07), P = 0.001b | 1.05 (1.01–1.10), P = 0.017b | |
| Coronary event | 1.17 (1.13–1.21), P < 0.001 | 1.20 (1.14–1.26), P < 0.001 |
| 1.02 (0.99–1.06), P = 0.213b | 1.09 (1.03–1.15), P = 0.003b | |
| Stroke | 1.17 (1.12–1.22), P < 0.001 | 1.19 (1.11–1.27), P < 0.001 |
| 0.98 (0.93–1.03), P = 0.488b | 1.06 (0.99–1.14), P = 0.111b | |
| Composite endpoint (CE, stroke, or death) | 1.18 (1.15–1.20), P < 0.001 | 1.17 (1.13–1.21), P < 0.001 |
| 1.04 (1.02–1.06), P = 0.001b | 1.05 (1.01–1.09), P = 0.011b | |
| Endpoint . | Systolic OBPRa . | Diastolic OBPRa . |
|---|---|---|
| All-cause mortality | 1.21 (1.18–1.23), P < 0.001 | 1.18 (1.13–1.22), P < 0.001 |
| 1.05 (1.02–1.07), P = 0.001b | 1.05 (1.01–1.10), P = 0.017b | |
| Coronary event | 1.17 (1.13–1.21), P < 0.001 | 1.20 (1.14–1.26), P < 0.001 |
| 1.02 (0.99–1.06), P = 0.213b | 1.09 (1.03–1.15), P = 0.003b | |
| Stroke | 1.17 (1.12–1.22), P < 0.001 | 1.19 (1.11–1.27), P < 0.001 |
| 0.98 (0.93–1.03), P = 0.488b | 1.06 (0.99–1.14), P = 0.111b | |
| Composite endpoint (CE, stroke, or death) | 1.18 (1.15–1.20), P < 0.001 | 1.17 (1.13–1.21), P < 0.001 |
| 1.04 (1.02–1.06), P = 0.001b | 1.05 (1.01–1.09), P = 0.011b | |
aOBPR effects are presented for 10 mmHg difference.
bCox proportional hazard model adjusted for age, gender, SBP, AHT, total cholesterol, diabetes, BMI, current smoking, previous CVD, and cancer (mortality only).
Discussion
Orthostatic hypotension has been traditionally associated with neurodegenerative diseases (e.g. Parkinson's disease, multiple system atrophy, or autonomic neuropathies),25 frail elderly patients3,26,27 and chronic heart failure.9,28,29 Furthermore, both hypertension30–32 and diabetes33,34 have been related to impaired orthostatic homeostasis through mechanisms, which are not yet completely understood. In such high-risk settings, OH demonstrates higher prevalence than in the general population, suggesting a multifactorial aetiology of postural haemodynamic failure. So the question arises when do OH-related disorders start in life and what are the main predisposing factors for developing OH?
To our knowledge, this is the largest longitudinal study exploring prevalence of OH in an unselected middle-aged urban population. In the MPP cohort, ∼6% of all individuals, aged between 28 and 61 years, predominantly free from advanced chronic diseases at baseline, met the criteria of OH. This prevalence is close to that reported in the ARIC study, where 5.1% of participants were found having OH.16 Similar to the ARIC study, age, hypertension, diabetes, current smoking, and history of CVD were related to OH. However, we found that OH may be also determined by female gender, antihypertensive treatment, and low BMI.
Factors predisposing for OH seem to be related to those for essential hypertension, CVD (history of CE and stroke, smoking) and metabolic disorders (diabetes, low BMI). Thus, OH may be a part of a continuum, which comprises well-known interrelated haemodynamic (hypertension), CVD, autonomic and metabolic (diabetes) disorders, still representing an independent risk factor for mortality and CE. Our study does not allow any conclusion as to whether OH is a causative risk factor or whether it is a secondary sign of subclinical disease. However, the fact that the OH relationship with mortality was strengthened in the younger segment of the population, in concordance with what was reported for the first time in the ARIC cohort,15–17 indicates that OH may be causatively related to increased risk of mortality. On the other hand, the relationship between OH and CE seemed to be similar in those aged ≥42 years, whereas in the youngest segment it was non-significant, which partly could be explained by less power in this group. The most definitive way to test whether OH has a causative relationship with increased mortality and CE risk, i.e. a randomized controlled interventional trial, is difficult to design to date as there are few, if any, pharmacological agents that conclusively have shown to improve an impaired orthostatic response. However, the results of our study and those of the ARIC study emphasize the need for development of novel pharmacological and non-pharmacological interventions for symptomatic and non-symptomatic OH.
Apart from the dichotomous variable of OH, we analysed OBPR as a continuous variable. Distribution of systolic and diastolic OBPR was normal and median values were near 0 mmHg, as expected. In crude Cox regression model, all endpoints were predicted by both variables. However, after adjustment only diastolic OBPR independently predicted higher CE incidence. As myocardial (especially subendocardial) perfusion occurs mainly during diastole,35 we can hypothesize that diurnal OH-related diastolic BP fluctuations influence coronary circulation to a higher degree than postural systolic BP changes. In fact, a connection between diastolic OH and CE was found in one study investigating home-dwelling elderly.13 Although symptomatic OH is usually characterized by falls in both systolic and diastolic BP, there was surprisingly little overlap (8.3%) between subjects with systolic OH and diastolic OH among our, presumably mainly asymptomatic, OH subjects, which further supports the fact that systolic and diastolic OH may represent different pathophysiological entities.
Our results confirm that impaired orthostatic haemodynamic response is associated with increased all-cause mortality and increased CE incidence, whereas a relationship to stroke remains uncertain. The latter can be partially explained by almost two times lower stroke incidence in comparison with CE incidence. The lower stroke incidence was, however, expected as it increases essentially first after the age of 65 years (1.83/1000 person-years in those aged 55–64 vs. 5.46/1000 person-years in those aged 65–7436), which can be confronted with median age of 45.7 years and mean follow-up time of 22.7 years in MPP. Although the associations between OH and all tested endpoints were attenuated after adjustment for traditional risk factors, a significant effect of baseline OH on overall mortality and CE incidence persisted. It can be further discussed which of the results—those from the ARIC study (relatively higher HRs) or those from the MPP cohort—better reflect reality but the main conclusion remains the same: OH confers not only higher risk for debilitating symptoms but also for earlier death and CE. A part of the explanation why the ARIC cohort demonstrated higher risks could be demographic and epidemiological differences between two cohorts: in the ARIC study, median age was higher (54 years), follow-up time shorter (13 years), there were more women (55%) and more participants with history of CVD (7%), cancer (6%), and diabetes (12%). It is worth noting, however, that those individuals who fulfilled more restrictive (i.e. higher cut-off limits) OH criteria demonstrated similar adjusted mortality and CE risk as OH positive individuals in the ARIC cohort. We can suppose that methodology of BP measurements in MPP was not sufficiently accurate at the time of recruitment to eliminate borderline cases. Consequently, inclusion of false OH positive individuals in statistical analysis might have attenuated the results.
In conclusion, OH can be easily detected and its prevalence in the middle-aged segment of the general population is close to 6%. The impaired orthostatic homeostasis is associated with age, female gender, low BMI, hypertension, antihypertensive treatment, higher heart rate, diabetes, and current smoking. Presence of OH signals increased risk of mortality and CE, independently of traditional risk factors, with mortality risk particularly accentuated in persons younger than 42 years. In addition, more pronounced orthostatic BP drop predicts higher mortality and higher incidence of CEs. Increased mortality in OH is related to both systolic and diastolic impairment, whereas incidence of CEs shows stronger association with diastolic BP fall. Further studies on pathological mechanisms underlying OH and potential therapeutic approaches are needed.
Funding
This work was supported by grants from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, the Medical Faculty of Lund University, Malmö University Hospital, the Albert Påhlsson Research Foundation, the Crafoord Foundation, the Ernhold Lundströms Research Foundation, the Region Skane, the Hulda and Conrad Mossfelt Foundation, the King Gustaf V and Queen Victoria Foundation, The Wallenberg Foundation and the Lennart Hanssons Memorial Fund. Funding to pay the Open Access publication charges for this article was provided by Lund University.
Conflict of interest: none declared.
References
- antihypertensive agents
- smoking
- hypertension
- body mass index procedure
- cardiovascular diseases
- diabetes mellitus
- systolic blood pressure
- diabetes mellitus, type 2
- orthostatic hypotension
- diastole
- follow-up
- middle-aged adult
- systole
- mortality
- gender
- coronary heart disease
- diastolic blood pressure
- cox proportional hazards models
- prevention