-
PDF
- Split View
-
Views
-
Cite
Cite
Grace Lin, Bernard J. Gersh, Eddie L. Greene, Margaret M. Redfield, David L. Hayes, Peter A. Brady, Renal function and mortality following cardiac resynchronization therapy, European Heart Journal, Volume 32, Issue 2, January 2011, Pages 184–190, https://doi.org/10.1093/eurheartj/ehq403
Close - Share Icon Share
Abstract
Cardiac resynchronization therapy (CRT) improves outcomes in heart failure, yet selection of patients likely to have survival benefit is problematic. Chronic kidney disease (CKD) is an important determinant of mortality in patients with congestive heart failure therefore we sought to determine the impact of CKD on mortality benefit after CRT.
All CRT device implantations in patients not on dialysis at Mayo Clinic between January 1999 and December 2005 were included. Of 482 patients, 342 (71%) had CKD (defined as a glomerular filtration rate (GFR) of ≤60 mL/min/1.73 m2) at the time of device implantation. Patients with CKD were older (71 ± 10 vs. 63 ± 14 years, P < 0.01) than patients without CKD, and more often anaemic (12.70 ± 1.73 vs. 13.24 mg/L, P < 0.01), with similar ejection fraction (22 ± 8 vs. 23 ± 8%, P = 0.32). Survival was superior in patients with normal or mild renal dysfunction compared with patients with CKD (72 vs. 57% at 3 years, P < 0.01). After multivariate analysis, CKD remained a significant predictor of poor survival following CRT.
Chronic kidney disease is common in patients undergoing CRT and associated with a higher mortality and should be considered when evaluating patients for CRT.
Introduction
Cardiac resynchronization therapy (CRT) improves outcomes in selected patients with advanced congestive heart failure (CHF), yet identification of individuals most likely to benefit from CRT remains problematic.1–3 Current selection of patients for CRT focuses on functional status along with evidence of electrical or mechanical dyssynchrony.2–5 Cardio-renal syndrome, renal dysfunction associated with CHF, confers a significantly worse prognosis independent of ejection fraction (EF) especially in cases that are severe enough to require dialysis. Unsurprisingly, severe chronic kidney disease (CKD), especially in patients requiring dialysis, is also associated with worse outcomes after implantable cardioverter defibrillator (ICD) and CRT.6–9 However, little is known regarding the impact of milder degrees of CKD on survival outcome following CRT since these patients have largely been excluded from CRT trials.1–3 This is important since close to 40% of patients with advanced CHF who may be candidates for CRT have CKD.10–15 Moreover, in most cases serum creatinine, a rather crude measure of renal function, has been used to diagnose CKD.16 We sought to determine whether milder forms of CKD that do not require dialysis, present at the time of CRT implantation, impact survival outcomes in a large cohort of patients undergoing CRT.
Methods
Patient selection
All consecutive patients who underwent implantation of a CRT device based on accepted clinical criteria between January 1999 and December 2005 at Mayo Clinic were included. Data, including demographics, echocardiographic data, laboratory values, hospitalization times, and medication use at the time of CRT device implantation were retrospectively obtained from a centralized clinical and medical record where all data were prospectively entered at the time of each medical visit or encounter, device implant, and subsequent follow-up. Patients were followed to a primary endpoint of death or need for heart transplantation. Additional data on vital statistics (death notification) and need for heart transplantation were obtained from questionnaires mailed to all patients.
Cardiac resynchronization therapy device implantation
Choice of a specific CRT device generator and leads, and decision to implant an ICD vs. CRT alone, was at the discretion of the operator in conjunction with the referring physician. Fluoroscopy times were used as a surrogate for total procedure length. In all cases, left ventricular pacing was initially attempted via the coronary sinus, unless a left ventricular epicardial lead had been placed previously. In circumstances where a coronary sinus lead could not be placed, or when a suitable endocardial site could not be found due to elevated pacing thresholds or persistent diaphragmatic stimulation, the decision to proceed with placement of a left ventricular epicardial lead via thoracotomy was made at the discretion of the primary clinician. In patients who were in sinus rhythm at the time of implantation, a right atrial lead was also placed. All devices were interrogated the day following surgery to confirm appropriate device function, lead stability, or the need for lead revision. Subsequent device interrogation and clinical evaluation were performed at 1 month and every 3 months thereafter. Patients who did not return for review and device interrogation at 1 month were considered lost to follow-up. All patients in whom a CRT device could not be implanted were excluded from the study analysis.
Definition of renal dysfunction
Renal function was measured at the time of CRT device implantation and characterized by glomerular filtration rate (GFR) estimate using the modification of diet in renal disease (MDRD) equation.17 Glomerular filtration rate values of >200 mL/min/1.73 m2 were set equal to 200 mL/min/1.73 m2.17 Patients with GFR ≥90 mL/min/1.73 m2 or 60–89 mL/min/1.73 m2 were considered to have normal or mildly decreased renal function, respectively. Chronic kidney disease was defined as a GFR of ≤60 mL/min/1.73 m2 (estimated by MDRD), and further classified as moderate (CKD stage III) if the GFR was 30–59 mL/min/1.73 m2, and severe (CKD stage IV) if the GFR was <30 mL/min/1.73 m2.16,18 For comparison, GFR was also estimated by the Cockgroft–Gault equation using both observed body weight (OBW) and ideal body weight (IBW) and indexed to body surface area.19 Patients receiving haemodialysis at the time of device implantation were excluded from the analysis.
Both acute and chronic changes in GFR following CRT device implantation were assessed. Acute change in GFR was defined as estimated GFR at first follow-up within 3 months following CRT. Chronic change in GFR was defined as estimated GFR at last follow-up. In all cases, both acute and chronic estimated GFRs were obtained during clinically indicated follow-up.
Statistical analysis
Differences in baseline, procedural, and follow-up characteristics between patients with and without CKD were compared using χ2, unpaired Student's t-test (two-sided) and Wilcoxon rank-sum tests. Mortality and need for heart transplantation in each group were assessed by Kaplan–Meier estimates and compared with the log-rank statistic. The independent predictive value of CKD on mortality or need for heart transplantation was determined using a Cox's proportional hazards regression model with consideration of other clinical variables previously shown to impact mortality and outcomes from CHF, including age, gender, aetiology of underlying cardiac disease, anaemia, hyponatraemia, EF, and B-type natriuretic peptide (BNP).20–24 Anaemia and hyponatraemia were defined using accepted clinical definitions (haemoglobin <12 g/dL and sodium <135 mmol/L). Age, EF, and BNP were analysed as dichotomous variables using the median value for each variable for the entire study population. A probability value of <0.05 was considered statistically significant.
All data collection and statistical analysis were performed by the primary investigators (G.L. and P.A.B.). Statistical analysis was performed using JMP 8.0 software (SAS).
This study was approved by and conducted in accordance with guidelines established by the Mayo Clinic Institutional Review Board.
Results
Patients
Between January 1999 and December 2005 a total of 512 patients underwent CRT device implantation. Implantation of a CRT device was possible via a lead placed within the coronary sinus in 486 patients (95%). An additional seven underwent subsequent placement of an epicardial lead. In the remaining 12 patients, CRT device implantation was not possible and these patients were excluded from further analysis. One patient without baseline creatinine measurement and ten patients with end stage renal disease receiving haemodialysis were also excluded.
Baseline renal function and clinical characteristics
Of the 482 patients who comprised the study population, more than two-thirds (342, 71%) had CKD as defined. Of these, the severity of CKD was moderate in 303 (63%) and severe in 39 (8%). In the 140 patients without CKD, 12 (2%) had normal renal function (GFR ≥90 mL/min/1.73 m2) and 128 (27%) had mildly decreased renal function or CKD stage II (GFR 60–89 mL/min/1.73 m2). Baseline GFR estimated by the MDRD and Cockgroft–Gault equations (by OBW and IBW) in each category are shown (Table 1). Cockgroft–Gault estimation of GFR could not be performed in 68 patients as exact weight at the time of CRT implant was not available. Estimation of GFR by Cockgroft–Gault was higher than MDRD estimation of GFR in all categories, and was the highest when OBW was used to estimate GFR (Table 1).
Baseline renal function
| GFR(mL/min/1.73 m2) . | No CKD* . | CKD* . | P-value . | ||
|---|---|---|---|---|---|
| . | Normal, n = 12 . | Mild, n = 128 . | Moderate, n = 303 . | Severe, n = 39 . | . |
| MDRD, n= 483 | 106.83 ± 23.36 | 69.54 ± 7.14 | 43.80 ± 10.44 | 25.41 ± 3.45 | < 0.01 |
| CG (OBW), n= 421 | 157.58 ± 37.14 | 97.10 ± 40.19 | 59.57 ± 24.50 | 33.18 ± 15.01 | < 0.01 |
| CG (IBW), n= 421 | 115.67 ± 28.42 | 69.79 ± 20.46 | 42.73 ± 15.95 | 24.17 ± 8.99 | < 0.01 |
| GFR(mL/min/1.73 m2) . | No CKD* . | CKD* . | P-value . | ||
|---|---|---|---|---|---|
| . | Normal, n = 12 . | Mild, n = 128 . | Moderate, n = 303 . | Severe, n = 39 . | . |
| MDRD, n= 483 | 106.83 ± 23.36 | 69.54 ± 7.14 | 43.80 ± 10.44 | 25.41 ± 3.45 | < 0.01 |
| CG (OBW), n= 421 | 157.58 ± 37.14 | 97.10 ± 40.19 | 59.57 ± 24.50 | 33.18 ± 15.01 | < 0.01 |
| CG (IBW), n= 421 | 115.67 ± 28.42 | 69.79 ± 20.46 | 42.73 ± 15.95 | 24.17 ± 8.99 | < 0.01 |
*Based on MDRD estimation of GFR. CG, Cockgroft–Gault.
Baseline renal function
| GFR(mL/min/1.73 m2) . | No CKD* . | CKD* . | P-value . | ||
|---|---|---|---|---|---|
| . | Normal, n = 12 . | Mild, n = 128 . | Moderate, n = 303 . | Severe, n = 39 . | . |
| MDRD, n= 483 | 106.83 ± 23.36 | 69.54 ± 7.14 | 43.80 ± 10.44 | 25.41 ± 3.45 | < 0.01 |
| CG (OBW), n= 421 | 157.58 ± 37.14 | 97.10 ± 40.19 | 59.57 ± 24.50 | 33.18 ± 15.01 | < 0.01 |
| CG (IBW), n= 421 | 115.67 ± 28.42 | 69.79 ± 20.46 | 42.73 ± 15.95 | 24.17 ± 8.99 | < 0.01 |
| GFR(mL/min/1.73 m2) . | No CKD* . | CKD* . | P-value . | ||
|---|---|---|---|---|---|
| . | Normal, n = 12 . | Mild, n = 128 . | Moderate, n = 303 . | Severe, n = 39 . | . |
| MDRD, n= 483 | 106.83 ± 23.36 | 69.54 ± 7.14 | 43.80 ± 10.44 | 25.41 ± 3.45 | < 0.01 |
| CG (OBW), n= 421 | 157.58 ± 37.14 | 97.10 ± 40.19 | 59.57 ± 24.50 | 33.18 ± 15.01 | < 0.01 |
| CG (IBW), n= 421 | 115.67 ± 28.42 | 69.79 ± 20.46 | 42.73 ± 15.95 | 24.17 ± 8.99 | < 0.01 |
*Based on MDRD estimation of GFR. CG, Cockgroft–Gault.
Baseline clinical characteristics of patients with and without CKD are shown (Table 2). Patients with CKD were older than patients without CKD (71 ± 10 vs. 63 ± 14 years, P < 0.01). Although baseline EF and severity of left ventricular enlargement were similar in patients with and without CKD (P = 0.20 and P = 0.24, respectively), ischaemic cardiomyopathy was more common in patients with CKD (P = 0.02).
Baseline clinical characteristics
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 342† . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 71 | 62 | 77 | 62 ± 14 | 71 ± 10 | <0.01 |
| Gender (male) | 115 (82%) | 269 (79%) | 0.38 | |||
| Ischaemic cardiomyopathy | 75 (54%) | 222 (65%) | 0.02 | |||
| Non-ischaemic cardiomyopathy | 65 (42%) | 120 (35%) | 0.02 | |||
| QRS duration (ms) | 164 | 140 | 188 | 161.49 ± 31.59 | 164.31 ± 33.80 | 0.40 |
| Ejection fraction (n= 136, n= 340) | 29 | 15 | 25 | 23 ± 8% | 22 ± 8% | 0.32 |
| LVEDD (mm) (n= 106, n= 252) | 66 | 61 | 73 | 68.17 ± 10.05 | 66.81 ± 9.47 | 0.23 |
| LVESD (mm) (n= 89, n= 204) | 58 | 53 | 66 | 60.30 ± 11.14 | 58.77 ± 9.81 | 0.26 |
| Hgb (mg/L) (n= 140, n= 360) | 12.90 | 11.50 | 14.00 | 13.24 ± 1.75 | 12.70 ± 1.73 | <0.01 |
| Sodium (mmol/L) (n= 137, n= 339) | 139 | 137 | 141 | 138.66 ± 3.29 | 139.08 ± 3.36 | 0.21 |
| BNP (ng/dL) (n= 68, n= 140) | 506.5 | 204.5 | 870.3 | 507.35 ± 620.59 | 778.64 ± 727.79 | <0.01 |
| Creatinine (mg/dL) | 1.4 | 1.2 | 1.7 | 1.07 ± 0.14 | 1.67 ± 0.46 | <0.01 |
| GFR | 51 | 39 | 62 | 72.74 ± 14.12 | 43.80 ± 10.44 | <0.01 |
| Beta-blockers | 111 (79%) | 274 (80%) | 0.84 | |||
| ACE-inhibitors/ARB | 121 (86%) | 289 (85%) | 0.58 | |||
| Diuretics | 113 (81%) | 316 (92%) | <0.01 | |||
| Nitrates | 17 (12%) | 73 (21%) | 0.02 | |||
| Digoxin | 80 (57%) | 225 (66%) | 0.08 | |||
| Calcium channel blockers | 9 (6%) | 14 (4%) | 0.29 | |||
| Amiodarone | 20 (14%) | 73 (21%) | 0.07 | |||
| Sotalol | 2 (1%) | 3 (1%) | 0.60 | |||
| Propafenone | 1 (1%) | 2 (1%) | 0.87 | |||
| Mexilitene | 3 (2%) | 4 (1%) | 0.43 | |||
| Quinidine | 0 (0%) | 2 (1%) | 0.24 |
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 342† . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 71 | 62 | 77 | 62 ± 14 | 71 ± 10 | <0.01 |
| Gender (male) | 115 (82%) | 269 (79%) | 0.38 | |||
| Ischaemic cardiomyopathy | 75 (54%) | 222 (65%) | 0.02 | |||
| Non-ischaemic cardiomyopathy | 65 (42%) | 120 (35%) | 0.02 | |||
| QRS duration (ms) | 164 | 140 | 188 | 161.49 ± 31.59 | 164.31 ± 33.80 | 0.40 |
| Ejection fraction (n= 136, n= 340) | 29 | 15 | 25 | 23 ± 8% | 22 ± 8% | 0.32 |
| LVEDD (mm) (n= 106, n= 252) | 66 | 61 | 73 | 68.17 ± 10.05 | 66.81 ± 9.47 | 0.23 |
| LVESD (mm) (n= 89, n= 204) | 58 | 53 | 66 | 60.30 ± 11.14 | 58.77 ± 9.81 | 0.26 |
| Hgb (mg/L) (n= 140, n= 360) | 12.90 | 11.50 | 14.00 | 13.24 ± 1.75 | 12.70 ± 1.73 | <0.01 |
| Sodium (mmol/L) (n= 137, n= 339) | 139 | 137 | 141 | 138.66 ± 3.29 | 139.08 ± 3.36 | 0.21 |
| BNP (ng/dL) (n= 68, n= 140) | 506.5 | 204.5 | 870.3 | 507.35 ± 620.59 | 778.64 ± 727.79 | <0.01 |
| Creatinine (mg/dL) | 1.4 | 1.2 | 1.7 | 1.07 ± 0.14 | 1.67 ± 0.46 | <0.01 |
| GFR | 51 | 39 | 62 | 72.74 ± 14.12 | 43.80 ± 10.44 | <0.01 |
| Beta-blockers | 111 (79%) | 274 (80%) | 0.84 | |||
| ACE-inhibitors/ARB | 121 (86%) | 289 (85%) | 0.58 | |||
| Diuretics | 113 (81%) | 316 (92%) | <0.01 | |||
| Nitrates | 17 (12%) | 73 (21%) | 0.02 | |||
| Digoxin | 80 (57%) | 225 (66%) | 0.08 | |||
| Calcium channel blockers | 9 (6%) | 14 (4%) | 0.29 | |||
| Amiodarone | 20 (14%) | 73 (21%) | 0.07 | |||
| Sotalol | 2 (1%) | 3 (1%) | 0.60 | |||
| Propafenone | 1 (1%) | 2 (1%) | 0.87 | |||
| Mexilitene | 3 (2%) | 4 (1%) | 0.43 | |||
| Quinidine | 0 (0%) | 2 (1%) | 0.24 |
*Based on MDRD estimation of GFR; †except when otherwise noted.
Baseline clinical characteristics
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 342† . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 71 | 62 | 77 | 62 ± 14 | 71 ± 10 | <0.01 |
| Gender (male) | 115 (82%) | 269 (79%) | 0.38 | |||
| Ischaemic cardiomyopathy | 75 (54%) | 222 (65%) | 0.02 | |||
| Non-ischaemic cardiomyopathy | 65 (42%) | 120 (35%) | 0.02 | |||
| QRS duration (ms) | 164 | 140 | 188 | 161.49 ± 31.59 | 164.31 ± 33.80 | 0.40 |
| Ejection fraction (n= 136, n= 340) | 29 | 15 | 25 | 23 ± 8% | 22 ± 8% | 0.32 |
| LVEDD (mm) (n= 106, n= 252) | 66 | 61 | 73 | 68.17 ± 10.05 | 66.81 ± 9.47 | 0.23 |
| LVESD (mm) (n= 89, n= 204) | 58 | 53 | 66 | 60.30 ± 11.14 | 58.77 ± 9.81 | 0.26 |
| Hgb (mg/L) (n= 140, n= 360) | 12.90 | 11.50 | 14.00 | 13.24 ± 1.75 | 12.70 ± 1.73 | <0.01 |
| Sodium (mmol/L) (n= 137, n= 339) | 139 | 137 | 141 | 138.66 ± 3.29 | 139.08 ± 3.36 | 0.21 |
| BNP (ng/dL) (n= 68, n= 140) | 506.5 | 204.5 | 870.3 | 507.35 ± 620.59 | 778.64 ± 727.79 | <0.01 |
| Creatinine (mg/dL) | 1.4 | 1.2 | 1.7 | 1.07 ± 0.14 | 1.67 ± 0.46 | <0.01 |
| GFR | 51 | 39 | 62 | 72.74 ± 14.12 | 43.80 ± 10.44 | <0.01 |
| Beta-blockers | 111 (79%) | 274 (80%) | 0.84 | |||
| ACE-inhibitors/ARB | 121 (86%) | 289 (85%) | 0.58 | |||
| Diuretics | 113 (81%) | 316 (92%) | <0.01 | |||
| Nitrates | 17 (12%) | 73 (21%) | 0.02 | |||
| Digoxin | 80 (57%) | 225 (66%) | 0.08 | |||
| Calcium channel blockers | 9 (6%) | 14 (4%) | 0.29 | |||
| Amiodarone | 20 (14%) | 73 (21%) | 0.07 | |||
| Sotalol | 2 (1%) | 3 (1%) | 0.60 | |||
| Propafenone | 1 (1%) | 2 (1%) | 0.87 | |||
| Mexilitene | 3 (2%) | 4 (1%) | 0.43 | |||
| Quinidine | 0 (0%) | 2 (1%) | 0.24 |
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 342† . | P-value . |
|---|---|---|---|---|---|---|
| Age (years) | 71 | 62 | 77 | 62 ± 14 | 71 ± 10 | <0.01 |
| Gender (male) | 115 (82%) | 269 (79%) | 0.38 | |||
| Ischaemic cardiomyopathy | 75 (54%) | 222 (65%) | 0.02 | |||
| Non-ischaemic cardiomyopathy | 65 (42%) | 120 (35%) | 0.02 | |||
| QRS duration (ms) | 164 | 140 | 188 | 161.49 ± 31.59 | 164.31 ± 33.80 | 0.40 |
| Ejection fraction (n= 136, n= 340) | 29 | 15 | 25 | 23 ± 8% | 22 ± 8% | 0.32 |
| LVEDD (mm) (n= 106, n= 252) | 66 | 61 | 73 | 68.17 ± 10.05 | 66.81 ± 9.47 | 0.23 |
| LVESD (mm) (n= 89, n= 204) | 58 | 53 | 66 | 60.30 ± 11.14 | 58.77 ± 9.81 | 0.26 |
| Hgb (mg/L) (n= 140, n= 360) | 12.90 | 11.50 | 14.00 | 13.24 ± 1.75 | 12.70 ± 1.73 | <0.01 |
| Sodium (mmol/L) (n= 137, n= 339) | 139 | 137 | 141 | 138.66 ± 3.29 | 139.08 ± 3.36 | 0.21 |
| BNP (ng/dL) (n= 68, n= 140) | 506.5 | 204.5 | 870.3 | 507.35 ± 620.59 | 778.64 ± 727.79 | <0.01 |
| Creatinine (mg/dL) | 1.4 | 1.2 | 1.7 | 1.07 ± 0.14 | 1.67 ± 0.46 | <0.01 |
| GFR | 51 | 39 | 62 | 72.74 ± 14.12 | 43.80 ± 10.44 | <0.01 |
| Beta-blockers | 111 (79%) | 274 (80%) | 0.84 | |||
| ACE-inhibitors/ARB | 121 (86%) | 289 (85%) | 0.58 | |||
| Diuretics | 113 (81%) | 316 (92%) | <0.01 | |||
| Nitrates | 17 (12%) | 73 (21%) | 0.02 | |||
| Digoxin | 80 (57%) | 225 (66%) | 0.08 | |||
| Calcium channel blockers | 9 (6%) | 14 (4%) | 0.29 | |||
| Amiodarone | 20 (14%) | 73 (21%) | 0.07 | |||
| Sotalol | 2 (1%) | 3 (1%) | 0.60 | |||
| Propafenone | 1 (1%) | 2 (1%) | 0.87 | |||
| Mexilitene | 3 (2%) | 4 (1%) | 0.43 | |||
| Quinidine | 0 (0%) | 2 (1%) | 0.24 |
*Based on MDRD estimation of GFR; †except when otherwise noted.
Biochemical markers at the time of CRT implantation are also shown (Table 2). Mean creatinine was higher and estimated GFR was lower in patients with CKD than in patients without CKD (P < 0.01 for both). However, in both patients with and without CKD, mean creatinine was <2 mg/dL at the time of CRT device implantation. Patients with CKD were mildly anaemic (mean haemoglobin 12.69 ± 1.77 g/dL) when compared with patients without CKD (13.24 ± 1.76 g/dL, P = 0.02). Hyponatraemia (serum sodium <135 mmol/L) was uncommon in either group of patients. Although serum BNP was increased in both patients with and without CKD, it was the highest in patients with CKD (P < 0.01).
All patients were receiving maximally tolerated medical therapy for heart failure at the time of CRT device implantation (Table 2). There was no difference in ACE-inhibitor or angiotensin receptor blocker use between the two groups; long-acting nitrates (22 vs. 12%, P < 0.01) and diuretics (91 vs. 80%, P = 0.02) were however used in a greater number of patients with CKD when compared with patients without CKD.
Procedural factors
Procedural data are summarized in Table 3. Patients without CKD were more likely to receive a CRT-defibrillator than patients with CKD (84 vs. 74%, P = 0.02). Total fluoroscopy time was shorter in patients without CKD compared with patients with CKD (41.81 ± 26.29 vs. 47.62 ± 29.43 min, P = 0.03), while length of hospitalization for CRT device implantation was similar in both groups.
Following cardiac resynchronization therapy
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 362† . | P-value . |
|---|---|---|---|---|---|---|
| Fluoroscopy times (min) (n= 139, n= 337) | 37.6 | 25 | 61 | 41.82 ± 26.28 | 47.55 ± 29.34 | 0.04 |
| Length of hospitalization (days) (n= 126, n= 298) | 1 | 1 | 5 | 4.47 ± 7.91 | 3.68 ± 4.83 | 0.30 |
| CRT-defibrillator | 118 (84%) | 267 (74%) | 0.02 | |||
| Ejection fraction (n= 96, n= 232) | 26 | 20 | 37 | 31 ± 15% | 29 ± 13% | 0.19 |
| LVEDD (mm) (n= 84, n= 209) | 63 | 56 | 71 | 64.55 ± 12.36 | 64.03 ± 10.89 | 0.74 |
| LVESD (mm) (n= 71, n= 179) | 55 | 44 | 63 | 55.95 ± 14.97 | 54.01 ± 12.86 | 0.34 |
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 362† . | P-value . |
|---|---|---|---|---|---|---|
| Fluoroscopy times (min) (n= 139, n= 337) | 37.6 | 25 | 61 | 41.82 ± 26.28 | 47.55 ± 29.34 | 0.04 |
| Length of hospitalization (days) (n= 126, n= 298) | 1 | 1 | 5 | 4.47 ± 7.91 | 3.68 ± 4.83 | 0.30 |
| CRT-defibrillator | 118 (84%) | 267 (74%) | 0.02 | |||
| Ejection fraction (n= 96, n= 232) | 26 | 20 | 37 | 31 ± 15% | 29 ± 13% | 0.19 |
| LVEDD (mm) (n= 84, n= 209) | 63 | 56 | 71 | 64.55 ± 12.36 | 64.03 ± 10.89 | 0.74 |
| LVESD (mm) (n= 71, n= 179) | 55 | 44 | 63 | 55.95 ± 14.97 | 54.01 ± 12.86 | 0.34 |
*Based on MDRD estimation of GFR.
†Except when otherwise noted.
Following cardiac resynchronization therapy
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 362† . | P-value . |
|---|---|---|---|---|---|---|
| Fluoroscopy times (min) (n= 139, n= 337) | 37.6 | 25 | 61 | 41.82 ± 26.28 | 47.55 ± 29.34 | 0.04 |
| Length of hospitalization (days) (n= 126, n= 298) | 1 | 1 | 5 | 4.47 ± 7.91 | 3.68 ± 4.83 | 0.30 |
| CRT-defibrillator | 118 (84%) | 267 (74%) | 0.02 | |||
| Ejection fraction (n= 96, n= 232) | 26 | 20 | 37 | 31 ± 15% | 29 ± 13% | 0.19 |
| LVEDD (mm) (n= 84, n= 209) | 63 | 56 | 71 | 64.55 ± 12.36 | 64.03 ± 10.89 | 0.74 |
| LVESD (mm) (n= 71, n= 179) | 55 | 44 | 63 | 55.95 ± 14.97 | 54.01 ± 12.86 | 0.34 |
| . | Median . | Lower quartile (25%) . | Upper quartile (75%) . | No CKD*, n = 140† . | CKD*, n = 362† . | P-value . |
|---|---|---|---|---|---|---|
| Fluoroscopy times (min) (n= 139, n= 337) | 37.6 | 25 | 61 | 41.82 ± 26.28 | 47.55 ± 29.34 | 0.04 |
| Length of hospitalization (days) (n= 126, n= 298) | 1 | 1 | 5 | 4.47 ± 7.91 | 3.68 ± 4.83 | 0.30 |
| CRT-defibrillator | 118 (84%) | 267 (74%) | 0.02 | |||
| Ejection fraction (n= 96, n= 232) | 26 | 20 | 37 | 31 ± 15% | 29 ± 13% | 0.19 |
| LVEDD (mm) (n= 84, n= 209) | 63 | 56 | 71 | 64.55 ± 12.36 | 64.03 ± 10.89 | 0.74 |
| LVESD (mm) (n= 71, n= 179) | 55 | 44 | 63 | 55.95 ± 14.97 | 54.01 ± 12.86 | 0.34 |
*Based on MDRD estimation of GFR.
†Except when otherwise noted.
Following cardiac resynchronization therapy
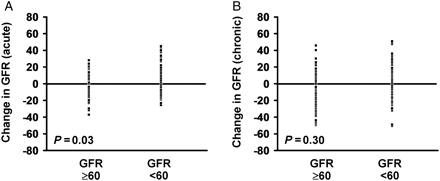
Follow-up echocardiographic data were available in 319 patients and were obtained at 36.88 ± 25.75 months following CRT implantation (Table 3). Ejection fraction improved and left ventricular end-diastolic dimension (LVEDD) decreased in both groups following CRT. There was no difference in the observed improvement of EF (9 ± 14 vs. 7 ± 12%, P = 0.15) or LVEDD (−3.16 ± 7.89 vs. −3.25 ± −7.77 mm, P = 0.94) amongst patients with and without CKD. Acute change in creatinine was measured and GFR estimated in 324 patients. Of these, creatinine was measured within 1 week of CRT device implantation in 243 patients, and in the remaining 81 patients, creatinine was measured between 1 and 3 months after the procedure. Estimated GFR improved modestly in patients with baseline CKD (mean change 0.95 ± 8.71 mL/min/1.73 m2) and decreased modestly in patients without baseline CKD (mean change GFR −1.99 ± 11.23 mL/min/1.73 m2, P = 0.03; Figure 1).
Acute (A) and Chronic (B) change in estimated glomerular filtration rate (mL/min/1.73 m2) following cardiac resynchronization therapy. Change in glomerular filtration rate in each individual patient is shown.
At long-term follow-up, 28 patients required haemodialysis; all of these patients had baseline CKD (mean estimated GFR 31.25 ± 12.90 mL/min/1.73 m2). Amongst the remaining patients, creatinine was measured and chronic change in GFR estimated in 320 patients (range 4–127 months, median 38 months following CRT device implantation). In patients with baseline CKD, estimated GFR was unchanged (mean change 0.2 ± 13.55 mL/min/1.73 m2), while GFR decreased in patients without baseline CKD (mean change −2.01 ± 16.95 mL/min/1.73 m2, P = 0.30; Figure 1), although this difference was not statistically significant.
Survival
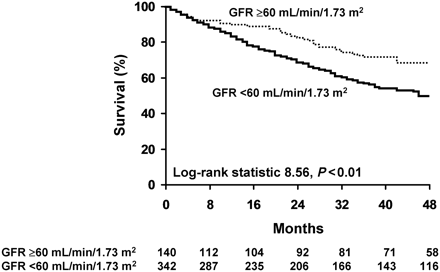
During a mean follow-up of 36.45 ± 26.55 months, 215 patients died and 27 required heart transplantation. Chronic kidney disease at the time of CRT implantation was associated with worse survival (Figure 2). Survival at 1 year of patients with normal (GFR ≥90 mL/min/1.73 m2) or mild CKD (GFR 60–90 mL/min/1.73 m2) was only slightly better (90 vs. 83%) than in patients with moderate CKD (GFR < 60 mL/min/1.73 m2). However, at 3 years, survival of patients with better preserved renal function was significantly higher (72 vs. 57%, P < 0.01). When patients who underwent heart transplantation were excluded from the analysis, survival at 3 years remained significantly better in patients without baseline CKD (74 vs. 60%, P < 0.01).
Survival following cardiac resynchronization therapy in patients with and without chronic kidney disease. Kaplan–Meier estimates of survival following cardiac resynchronization therapy device implantation in patients with estimated glomerular filtration rate ≥60 mL/min/1.73 m2 (normal or mild renal dysfunction; no chronic kidney disease) and with estimated glomerular filtration rate <60 mL/min/1.73 m2 (moderate or severe renal dysfunction; chronic kidney disease present).
In univariate analysis, CKD was associated with significantly worse outcome (HR 1.59; 95% CI 1.17–2.20, P < 0.01, Table 4). Anaemia (Hgb <12 mg/L), hyponatraemia (sodium <135 mmol/L), increased BNP (>500 ng/dL), male gender, and EF <20% were also predictive of poor outcomes (death or need for heart transplantation). Non-ischaemic cardiomyopathy was associated with lower mortality. After multivariate analysis, CKD remained a significant predictor of poor outcome, although anaemia (Hgb < 12.0 mg/dL) was the most robust predictor. Non-ischaemic cardiomyopathy remained a significant predictor of improved outcomes following CRT. Although the serum BNP level was a significant univariate predictor of worse mortality, it was only measured in 208 patients and therefore was not included in the multivariate analysis.
Univariate and multivariate risk analyses of mortality following cardiac resynchronization therapy
| n = 502 . | Hazard ratio . | 95% CI . | χ2 . | P-value . |
|---|---|---|---|---|
| Univariate | ||||
| Age >71 | 1.25 | 0.96–1.62 | 2.92 | 0.09 |
| Gender (male) | 1.91 | 1.34–2.83 | 13.43 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.54 | 0.40–0.72 | 18.50 | <0.01 |
| Ejection fraction <20% | 1.44 | 1.10–1.99 | 7.30 | <0.01 |
| Hgb <12.0 mg/L | 1.82 | 1.39–2.38 | 18.12 | <0.01 |
| Sodium <135 mmol/L | 1.96 | 1.24–2.88 | 10.05 | <0.01 |
| BNP >500 ng/dL | 2.82 | 1.81–4.52 | 21.82 | <0.01 |
| Creatinine (0.1 mg/dL increments) | 9.25 | 3.36–23.30 | 16.98 | <0.01 |
| Creatinine (>2 mg/dL) | 1.61 | 1.12–2.27 | 6.49 | 0.01 |
| GFR (MDRD<60 mL/min/1.73 m2) | 1.59 | 1.17–2.20 | 9.02 | <0.01 |
| GFR (CG, OBW <60) (n= 435) | 1.36 | 1.03–1.80 | 4.62 | 0.03 |
| GFR (CG, IBW <60) (n= 435) | 0.75 | 0.39 | ||
| Multivariate | ||||
| Gender (male) | 1.82 | 1.24–2.77 | 9.84 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.61 | 0.45–0.85 | 9.59 | <0.01 |
| EF (<20%) | 1.31 | 1.01–1.73 | 4.00 | 0.05 |
| GFR (MDRD <60 mL/min/1.73 m2) | 1.61 | 1.16–2.28 | 8.50 | <0.01 |
| Hgb (<12 mg/L) | 1.91 | 1.44–2.50 | 19.94 | <0.01 |
| Na (<135 mmol/L) | 1.76 | 1.16–2.56 | 6.85 | <0.01 |
| n = 502 . | Hazard ratio . | 95% CI . | χ2 . | P-value . |
|---|---|---|---|---|
| Univariate | ||||
| Age >71 | 1.25 | 0.96–1.62 | 2.92 | 0.09 |
| Gender (male) | 1.91 | 1.34–2.83 | 13.43 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.54 | 0.40–0.72 | 18.50 | <0.01 |
| Ejection fraction <20% | 1.44 | 1.10–1.99 | 7.30 | <0.01 |
| Hgb <12.0 mg/L | 1.82 | 1.39–2.38 | 18.12 | <0.01 |
| Sodium <135 mmol/L | 1.96 | 1.24–2.88 | 10.05 | <0.01 |
| BNP >500 ng/dL | 2.82 | 1.81–4.52 | 21.82 | <0.01 |
| Creatinine (0.1 mg/dL increments) | 9.25 | 3.36–23.30 | 16.98 | <0.01 |
| Creatinine (>2 mg/dL) | 1.61 | 1.12–2.27 | 6.49 | 0.01 |
| GFR (MDRD<60 mL/min/1.73 m2) | 1.59 | 1.17–2.20 | 9.02 | <0.01 |
| GFR (CG, OBW <60) (n= 435) | 1.36 | 1.03–1.80 | 4.62 | 0.03 |
| GFR (CG, IBW <60) (n= 435) | 0.75 | 0.39 | ||
| Multivariate | ||||
| Gender (male) | 1.82 | 1.24–2.77 | 9.84 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.61 | 0.45–0.85 | 9.59 | <0.01 |
| EF (<20%) | 1.31 | 1.01–1.73 | 4.00 | 0.05 |
| GFR (MDRD <60 mL/min/1.73 m2) | 1.61 | 1.16–2.28 | 8.50 | <0.01 |
| Hgb (<12 mg/L) | 1.91 | 1.44–2.50 | 19.94 | <0.01 |
| Na (<135 mmol/L) | 1.76 | 1.16–2.56 | 6.85 | <0.01 |
Univariate and multivariate risk analyses of mortality following cardiac resynchronization therapy
| n = 502 . | Hazard ratio . | 95% CI . | χ2 . | P-value . |
|---|---|---|---|---|
| Univariate | ||||
| Age >71 | 1.25 | 0.96–1.62 | 2.92 | 0.09 |
| Gender (male) | 1.91 | 1.34–2.83 | 13.43 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.54 | 0.40–0.72 | 18.50 | <0.01 |
| Ejection fraction <20% | 1.44 | 1.10–1.99 | 7.30 | <0.01 |
| Hgb <12.0 mg/L | 1.82 | 1.39–2.38 | 18.12 | <0.01 |
| Sodium <135 mmol/L | 1.96 | 1.24–2.88 | 10.05 | <0.01 |
| BNP >500 ng/dL | 2.82 | 1.81–4.52 | 21.82 | <0.01 |
| Creatinine (0.1 mg/dL increments) | 9.25 | 3.36–23.30 | 16.98 | <0.01 |
| Creatinine (>2 mg/dL) | 1.61 | 1.12–2.27 | 6.49 | 0.01 |
| GFR (MDRD<60 mL/min/1.73 m2) | 1.59 | 1.17–2.20 | 9.02 | <0.01 |
| GFR (CG, OBW <60) (n= 435) | 1.36 | 1.03–1.80 | 4.62 | 0.03 |
| GFR (CG, IBW <60) (n= 435) | 0.75 | 0.39 | ||
| Multivariate | ||||
| Gender (male) | 1.82 | 1.24–2.77 | 9.84 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.61 | 0.45–0.85 | 9.59 | <0.01 |
| EF (<20%) | 1.31 | 1.01–1.73 | 4.00 | 0.05 |
| GFR (MDRD <60 mL/min/1.73 m2) | 1.61 | 1.16–2.28 | 8.50 | <0.01 |
| Hgb (<12 mg/L) | 1.91 | 1.44–2.50 | 19.94 | <0.01 |
| Na (<135 mmol/L) | 1.76 | 1.16–2.56 | 6.85 | <0.01 |
| n = 502 . | Hazard ratio . | 95% CI . | χ2 . | P-value . |
|---|---|---|---|---|
| Univariate | ||||
| Age >71 | 1.25 | 0.96–1.62 | 2.92 | 0.09 |
| Gender (male) | 1.91 | 1.34–2.83 | 13.43 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.54 | 0.40–0.72 | 18.50 | <0.01 |
| Ejection fraction <20% | 1.44 | 1.10–1.99 | 7.30 | <0.01 |
| Hgb <12.0 mg/L | 1.82 | 1.39–2.38 | 18.12 | <0.01 |
| Sodium <135 mmol/L | 1.96 | 1.24–2.88 | 10.05 | <0.01 |
| BNP >500 ng/dL | 2.82 | 1.81–4.52 | 21.82 | <0.01 |
| Creatinine (0.1 mg/dL increments) | 9.25 | 3.36–23.30 | 16.98 | <0.01 |
| Creatinine (>2 mg/dL) | 1.61 | 1.12–2.27 | 6.49 | 0.01 |
| GFR (MDRD<60 mL/min/1.73 m2) | 1.59 | 1.17–2.20 | 9.02 | <0.01 |
| GFR (CG, OBW <60) (n= 435) | 1.36 | 1.03–1.80 | 4.62 | 0.03 |
| GFR (CG, IBW <60) (n= 435) | 0.75 | 0.39 | ||
| Multivariate | ||||
| Gender (male) | 1.82 | 1.24–2.77 | 9.84 | <0.01 |
| Non-ischaemic cardiomyopathy | 0.61 | 0.45–0.85 | 9.59 | <0.01 |
| EF (<20%) | 1.31 | 1.01–1.73 | 4.00 | 0.05 |
| GFR (MDRD <60 mL/min/1.73 m2) | 1.61 | 1.16–2.28 | 8.50 | <0.01 |
| Hgb (<12 mg/L) | 1.91 | 1.44–2.50 | 19.94 | <0.01 |
| Na (<135 mmol/L) | 1.76 | 1.16–2.56 | 6.85 | <0.01 |
Discussion
The main finding of this study is that renal dysfunction at the time of CRT device implantation is common and even when only moderately impaired is associated with worse survival outcomes when compared with patients with preserved renal function. Mortality in patients with CKD was higher than in patients without CKD (83 vs. 90% at 1 year and 56 vs. 70% at 3 years) suggesting that haemodynamic improvement after CRT may have less impact on mortality in this patient group. Based on these findings, renal function, as determined by estimated GFR, should be considered when evaluating patients for CRT.
Prior studies have also reported worse outcomes following ICD and CRT in patients with end stage renal disease as determined by increased baseline serum creatinine.8,9 However, serum creatinine is a crude indicator of the presence of renal dysfunction, especially in the elderly, as it may be influenced by age-related changes in muscle mass.9,16 For example, in our study, we observed that mean creatinine of patients undergoing CRT with CKD was <2.0 mg/dL implying only mild or moderate renal dysfunction rather than more severe renal dysfunction (CKD stage III) as estimated by GFR. Moreover, we also found significant variation in classification of CKD when applying the Cockgroft–Gault or the MDRD formulas to estimate GFR, similar to a previous study which has demonstrated a higher accuracy of the MDRD equation to estimate renal function in patients with advanced heart failure compared with other methods.25 Thus, the use of serum creatinine as a measure of renal function in patients with heart failure who might be candidates for CRT would underestimate the prevalence of renal dysfunction hiding the incremental adverse prognostic impact of even mild forms of renal dysfunction in this population.
The worse prognosis of patients with CKD undergoing CRT observed in this study is not explained by the lack of favourable ventricular remodelling since both patients with and without CKD had similar increase in EF and decrease in left ventricular end-diastolic dimensions suggesting similar degrees of left ventricular reverse remodelling. Thus, the mere presence of even mild forms of CKD rather than the lack of favourable ventricular remodelling had a negative impact, or was a marker of worse outcome, after CRT. Our finding that anaemia, a frequent consequence of impaired renal function, was the most robust predictor of worse mortality after CRT, further supports the notion that renal function is an important predictor of mortality benefit following CRT.21,24
Although an early and/or transient deterioration in renal function may occur due to intravenous contrast exposure at the time of device implantation,26 we did not observe any clinically important change in GFR either acutely or during long-term follow-up. However, although GFR did not decrease dramatically in most patients either with or without CKD, of the 39 patients with stage IV CKD, over 70% eventually required haemodialysis. This finding suggests that although CRT can be safely performed in most patients with CKD it has a limited impact on delaying or preventing deterioration of renal function. Rather, our findings are consistent with previous observations that worsening renal function confers worse outcomes in heart failure,13–15 a phenomenon which remains apparent even after CRT.
Although outcomes in patients with moderate or severe CKD are worse compared with those with milder renal dysfunction, whether CRT provides any additional mortality benefit in this population beyond usual medical therapy for heart failure cannot be adequately determined from this study. Cardiac resynchronization therapy is now part of the accepted standard of care for patients with heart failure and is widely utilized in this patient population, and therefore we could not directly compare outcomes in patients with CKD treated with CRT to an adequate selection of those who are not treated with CRT. Recent data have argued for earlier placement of a CRT device in patients with minimal symptoms of heart failure to promote ventricular reverse remodelling and improved haemodynamics.27,28 Whether early placement of CRT will also translate into delayed onset of moderate CKD, and potential for greater mortality benefit, deserves further study.
Limitations
Owing to retrospective design, follow-up was limited to clinically indicated evaluations and therefore timing of echocardiographic measurements and GFR following CRT was not uniform and would include patients in various stages of response to CRT. Second, our study did not address other potential mechanisms for the observed increased mortality after CRT in patients with CKD, including the possibility that since fewer patients with CKD received a CRT-defibrillator, the higher mortality observed may have been a consequence of a higher rate of sudden cardiac arrest, which is supported by a recent post-hoc analysis of the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial that found a higher risk of sudden cardiac arrest in patients with renal dysfunction.29 However, the precise effect of CRT on the occurrence of ventricular arrhythmias and sudden cardiac arrest remains unclear.29,30
Conclusions
Moderate CKD at the time of CRT implantation is common and is associated with worse survival outcome. Serum creatinine as an indicator of the severity of renal dysfunction is unreliable when compared with measures of GFR and may underestimate the presence and severity of CKD.
Conflict of interest: Dr D.L.H. has the following conflicts: Educational speaking; Medtronic, Boston Scientific, St Jude Medical, Sorin/ELA, Sponsored research; Medtronic, Boston Scientific, St Jude Medical, Steering committee; Medtronic, Advisory board; Boston Scientific, Sorin/ELA, St Jude Medical. None of the other authors has any conflicts.