-
PDF
- Split View
-
Views
-
Cite
Cite
Peter van der Meer, Dirk J. van Veldhuisen, To bind or not to bind: potassium-lowering drugs in heart failure, European Heart Journal, Volume 32, Issue 7, April 2011, Pages 791–792, https://doi.org/10.1093/eurheartj/ehr058
Close - Share Icon Share
This editorial refers to ‘Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial’†, by B. Pitt et al., on page 820
More than a decade ago the first large randomized controlled trial studying the effects of spironolactone in heart failure patients was published.1,2 The Randomized Aldactone Evaluation Study (RALES) trial showed for the first time the robust effects of aldosterone receptor blockers on reducing mortality. In this landmark trial patients with systolic heart failure in New York Heart Association (NYHA) class III were included. It is well known that one of the potential serious side effects of spironolactone is hyperkalaemia. Therefore patients with potassium levels >5 mmol/L were excluded from participation in RALES and potassium levels were measured frequently. Probably because of these intense safety checks the incidence of serious hyperkalaemia was extremely low and occurred in only 2% of the patients randomized to spironolactone compared with 1% in the placebo group. Similar findings were observed in the EPHESUS trial, which studied the effects of eplerenone, a selective aldosterone receptor blocker, in myocardial infarction complicated by heart failure.3 Blocking the aldosterone receptor resulted in a significant reduction in all-cause mortality. However, serious hyperkalaemia (>6.0 mmol/L) rates were markedly higher in EPHESUS: 5.5% in the eplerenone group compared with 3.9% in the placebo group. The risk of serious hyperkalaemia was significantly increased among patients who had a decreased glomerular filtration rate (GFR) at baseline (<50 mL/min). There were no deaths in the eplerenone group that were attributed to hyperkalaemia. The third large trial with aldosterone blockers in heart failure was recently published. This Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) study was performed in patients with mild systolic heart failure in NYHA class II.4,5 This trial was stopped early, since eplerenone treatment resulted in a reduced risk of death and hospitalization. Hyperkalaemia (>6.0 mmol/l) was seen in 2.5% of the patients treated with eplerenone vs. 1.9% in the placebo group. Again patients with potassium levels >5.0 mmol/L and GFR <30 mL/min at baseline were excluded from participation.
Taking the results of the three landmark trials together, even in the controlled circumstances of a randomized trial, episodes of serious hyperkalaemia are relatively frequently observed. However, in these trials this did not result in deaths attributable to the use of aldosterone receptor antagonists; in real life with most likely fewer laboratory measurements, this may well be different. After the initial publication of the RALES trial Juurlink and colleagues6 studied the prescription rates of spironolactone and linked this to hospital admissions in more than one million patients. One of the most striking findings was the abrupt increase in hospitalizations for hyperkalaemia and subsequent death associated with this. Mortality rose from 0.3 per 1000 patients to 2.0 per 1000 patients. Although this was an observational study, the findings suggest that prudence is warranted when spironolactone is prescribed in heart failure patients especially in those with reduced kidney function and pre-existing elevated potassium levels.
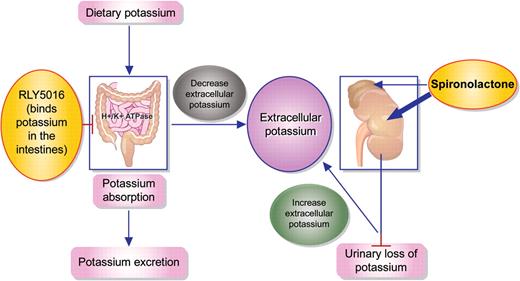
In the light of these findings, the study by Pitt et al.7 in the present issue of the Journal may be important. These authors performed a double-blind placebo-controlled trial to study the safety and efficacy of a potassium binder (RLY5016) on serum potassium in heart failure patients receiving spironolactone. This drug, which does not have a generic name yet, is a non-absorbed polymer binding potassium and increasing faecal potassium excretion (Figure 1). The authors included 104 chronic heart failure patients, who were randomized in a 1:1 fashion. Patients were eligible if they had either a history of hyperkalaemia that resulted in discontinuation of heart failure medication or chronic renal failure (GFR < 60 ml/min). The average left ventricular ejection fraction was ∼40%, and patients with either a reduced or a preserved ejection fraction could be included in the study. The use of RLY5016 decreased potassium levels by 0.22 mmol/L, whereas placebo resulted in an increase of 0.23 mmol/L. As expected, the incidence of hyperkalaemia was significantly lower in the RLY5016 group compared with placebo, 25% versus 7%, respectively. On the other hand, hypokalaemia occurred in 6% of the patients in the RLY5016 group, compared with none in the placebo group. This is of some concern. Hypokalaemia has been frequently associated with hypomagnesaemia, which may lead to arrhythmias. None were observed in the current study, but this may be attributable to its relatively small sample size. Furthermore, as with other potassium binding agents, RLY5016 is also associated with gastrointestinal side effects. Patients randomized to the RLY5016 arm had significantly more gastrointestinal adverse events compared with those randomized to placebo (21% vs. 6%). Although in this trial similar rates of drug discontinuation were seen between placebo and RLY5016 (6% and 7%, respectively), the duration of the trial was only 4 weeks. These gastrointestinal effects may limit to a certain extent the routine long-term use of this agent. Also, the effect of the drug may be more prominent in patients with severe renal failure, since they are most at risk for serious hyperkalaemia. Only four patients with severe renal failure (GFR < 30 ml/min) participated in the trial and the average renal function was 81 ml/min. This underscores that patients with relatively mild heart failure were included.8 Also, several trials are currently being executed to study the effect of spironolactone in patients with a preserved ejection fraction. 9,10 If treatment with spironolactone is indeed shown to be beneficial in this population, which accounts for half of the heart failure population, it will lead to substantially more frequent use of the drug. Therefore future studies with RLY5016 may be necessary to delineate the possible effects in specific cohorts of patients, including those with a preserved ejection fraction and with severe chronic kidney disease.
Use of spironolacton increases extracellular potassium levels; RLY5016 binds potassium in the intestines leading to less absorption and thereby lowering extracellular potassium levels.
In conclusion RLY5016 is a novel potassium binder that lowers potassium levels in patients with heart failure and spironolactone use. While larger randomized placebo-controlled trials are necessary in order to confirm that lowering potassium levels will indeed result in the use of higher dosages of renin–angiotensin–aldosterone system inhibitors, thereby possibly favourably affecting outcome, the results of Pitt and colleagues are an interesting first step in this direction.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
doi:10.1093/eurheartj/ehq502