-
PDF
- Split View
-
Views
-
Cite
Cite
Luigi M. Biasucci, Fulvio Bellocci, Maurizio Landolina, Roberto Rordorf, Antonello Vado, Endrj Menardi, Giovanna Giubilato, Serafino Orazi, Massimo Sassara, Antonello Castro, Riccardo Massa, Antoine Kheir, Gabriele Zaccone, Catherine Klersy, Francesco Accardi, Filippo Crea, Risk stratification of ischaemic patients with implantable cardioverter defibrillators by C-reactive protein and a multi-markers strategy: results of the CAMI-GUIDE study, European Heart Journal, Volume 33, Issue 11, June 2012, Pages 1344–1350, https://doi.org/10.1093/eurheartj/ehr487
Close - Share Icon Share
Abstract
Patients at risk of sudden cardiac death (SCD) after myocardial infarction (MI) can be offered therapy with implantable cardioverter defibrillators (ICDs). Whether plasma biomarkers can help risk stratify for SCD and ventricular arrhythmias (VT/VF) is unclear.
The primary objective of the CAMI-GUIDE study is to assess the predictive role of C-reactive protein for SCD or VT/VF in ischaemic patients with the ejection fraction <30% and ICDs. Secondary endpoints included all-cause mortality, hospitalizations, and death from heart failure. Additional analyses incorporated cystatin-C and NT-ProBNP in multi-marker approach for the prediction of adverse outcomes. A total of 300 patients were enrolled. All-cause mortality at 2 years was 22.6%, mortality from heart failure was 8.3%. Primary endpoint occurred in 17.3%. At a competing risk multivariable analysis adjusted for baseline variables, no significant difference in primary endpoint was found between patients with C-reactive protein ≤3 vs. >3 mg/L [heart rate (HR) 0.91 (0.50–1.64) P = 0.76], while C-reactive protein >3 mg/L was strongly associated with mortality due to heart failure [HR: 3.17 (1.54–6.54) P = 0.002]. NT-proBNP above median was significantly associated with the primary endpoint [adjusted HR: 1.46 (1.020–2.129) P = 0.042]. A risk function, including the three biomarkers, NYHA class and resting HR, allowed stratification of patient mortality risk from 5 to 50%.
C-reactive protein >3 mg/L is not associated with SCD or fast VT/VF, however, is a strong predictor of HF mortality. Biomarkers combined with clinical markers allow an excellent risk stratification of mortality at 2 years.
Introduction
Several clinical trials have shown that implantable cardioverter defibrillators (ICDs) are effective means for the prevention of sudden cardiac death (SCD) in both ischaemic and non-ischaemic patients.1–6 However, despite current evidence-based guidelines, ICDs still remain underused or misused, with implantation rates varying from country to country.7–9 Notably, patients with ischaemic heart disease and low left ventricular ejection fraction (LVEF) are exposed to an increased risk of SCD10 and although international guidelines were updated, years ago, to include this population into a Class IA indication for ICD implantation,11 criticisms still remain. In particular, whether or not all patients with a previous myocardial infarction (MI) and low LVEF should receive an ICD12–15 still remains an issue prompting debate and attempts are being made to better risk stratify these patients.
Although re-entry mechanisms have been considered the primary cause of ventricular arrhythmias in post-MI patients, recent findings support the hypothesis that acute ischaemia might also represent a prevalent mechanism of sudden death in post-MI, through different mechanisms, from increased release of cytokines, changes in metabolic and ions substrate to inhomogeneous conduction delays and blocks that may also, in turn, facilitate re-entry.16–19
The hypothesis behind the study was that different mechanisms underlay the genesis of arrhythmias in these patients and that acute ischaemia may play a dominant role in the occurrence of SCD or in the most fast and life-threatening forms of ventricular arrhythmias [i.e. ventricular fibrillation (VF) or fast ventricular tachycardia (VT)], while re-entry could be mainly associated to slower arrhythmias. Recently, specific plasma biomarkers have been introduced for risk stratification of acute coronary events (e.g. inflammatory cytokines, biomarkers of plaque destabilization and rupture, biomarkers of myocardial stretch).20
Therefore, the aim of the CAMI-GUIDE (C-reactive protein Assessment post Myocardial Infarction to GUIde DEfibrillator implantation) study was to prospectively test the hypothesis that plasma levels of the prototypic inflammatory biomarker C-reactive protein were associated with the risk of potentially fatal arrhythmias and SCD in a population of ischaemic patients with previous MI and LVEF <30% who received an ICD according to the inclusion criteria of the MADIT II study.3 Additionally, NT-proBNP and cystatin-C were also assessed in order to evaluate the potential additive benefit of a multi-marker approach for risk stratification.
Methods
CAMI-GUIDE was a multicentre prospective observational study involving 24 Italian centres experienced in ICD implantation and follow-up. Patients were enrolled between 15 October 2004 and 7 June 2006. The study design, including details on selection criteria, study endpoints, clinical follow-up and sample size, have been previously published.21 Briefly, the study enrolled patients with a previous MI (>30 days) and LVEF ≤30%, who were candidates for the implantation of an ICD or cardiac resynchronization therapy device with a defibrillator (CRT-D). The primary endpoint was to assess the relationship between C-reactive protein levels recorded at baseline and a combined endpoint of SCD or the occurrence of fast VT (>200 b.p.m.) or VF requiring ICD interventions at 2 years of follow-up. In particular, the sample was selected to detect an increase in at least 48.6% in the primary outcome in the subgroup of patients with C-reactive protein >3mg/L compared with those with C-reactive protein ≤3 mg/L. Secondary endpoints included all-cause mortality, heart failure (HF) hospitalizations, and HF-related mortality according to baseline C-reactive protein values. In addition to C-reactive protein, NT-proBNP and cystatin-C values were assessed in a parallel analysis to determine the additive prognostic value of a multi-marker approach for determining the risk of SCD or fast VT/VF and of all-cause mortality.
Statistical analysis
Descriptive statistics are presented for all collected variables, overall and according to pre-specified C-reactive protein groups. The Fisher exact test has been used to compare proportions and either the Student t-test or the Mann–Whitney U test to compare variables on a continuous scale. The association of C-reactive protein (≤/>3 mg/L) with the primary endpoint was assessed by means of Cox modelling. We chose for C-reactive protein a cut-off of 3 mg/L according to data and recommendation in literature on stable patients with ischaemic heart disease.22–24 The primary analysis was done in univariate fashion. Heart rate (HR) (95% CI) is presented together with Kaplan–Meier cumulative survival and with event rates (per 100 person year) in each group. Additionally, a multivariate analysis for competing events including available baseline parameters (cystatin-C, NTproBNP, LVEF, HR, NYHA class, QRS width, diabetes, previous revascularization, dilated post-ischaemic aetiology, peripheral artery disease, history of stroke, and current smoker) was performed in order to assess the role of C-reactive protein for each component of death: primary endpoint (SCD/VF/fastVT), HF-related death and death for other causes. The association of C-reactive protein with the endpoint was also analysed on a continuous scale and categorized according to quartiles of their distribution. Competing events (death from other causes, transplant or ventricular-assist device implant, myocardial infarction, and revascularization) were censored at their time of occurrence. Similar survival analysis techniques were used to evaluate time to event for the secondary endpoints, and to assess the predictive role of NT-proBNP and cystatin-C.
To evaluate the potential benefit of a multivariable approach for risk stratification, including both serum biomarkers and baseline clinical parameters, a multivariable Cox model was used to analyse the predictive value for all-cause mortality including the three considered biomarkers (PCR; Cys-C; NT-Pro BNP) and those clinical variables collected in the study, significantly linked at univariate analysis or of clinical interest and not collinear. Backward stepwise selection (P to remove >0.1) was performed to obtain a parsimonious model for risk stratification. This was performed by categorizing the predictor index (the linear combination of the predictors in the model) into the tertiles of its distribution. The Harrell's c-statistic for discrimination was computed. Stata 11 (StataCorp, College Station, TX, USA) was used for computation. All tests were two-sided and a P-value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 300 patients were recruited during the study. Sixteen patients were excluded from analysis for inclusion criteria violations: PTCA or CABG within 1 or 3 months from implant, respectively (6 patients), LVEF >30% assessed at baseline echocardiogram (five patients), acute MI within 1 month from implant (five patients). One additional patient was excluded for ICD implant refusal after having signed the informed consent. Fifteen patients did not have baseline blood samples drawn and were excluded from the analysis. Finally, 268 patients were considered for the final analysis, which exceeded the predefined minimum number of patients (252) needed to achieve adequate statistical power.21 One hundred twenty-two patients were implanted with a single-chamber ICD, 51 with dual-chamber ICD, and 94 with a CRT device. One patient was implanted with a dual chamber pacemaker instead of a CRT device due to refusal to ICD after CRT implant failure due to adverse coronary sinus anatomy. According to intention to treat analysis, the patient was included into the data set. One hundred forty-four patients (52.2%) had C-reactive protein at enrolment >3 mg/L. The baseline characteristics of the patients included in the data set (Table 1) were not statistically different between groups according to C-reactive protein≤3 or >3 mg/L, with the exception of NYHA class and resting HR, that were higher in the C-reactive protein >3 mg/L group.
Baseline demographics for patients enrolled in the study, overall and per C-reactive protein levels ≤3/>3
| Variable . | All patients (n = 276) . | C-reactive protein ≤3 (n = 124) . | C-reactive protein >3 (n = 144) . | P-value . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 67 ± 10 | 67 ± 10 | 68 ± 10 | 0.17 |
| LVEF (%, mean ± SD) | 26 ± 4 | 26 ± 4 | 26 ± 5 | 0.54 |
| Systolic blood pressure (mmHg; mean ± SD) | 120 ± 18 | 118 ± 17 | 122 ± 18 | 0.12 |
| Diastolic blood pressure (mmHg; mean ± SD) | 75 ± 10 | 74 ± 9 | 75 ± 10 | 0.13 |
| Resting heart rate (b.p.m., mean ± SD) | 72 ± 14 | 69 ± 12 | 74 ± 16 | 0.005 |
| QRS width (ms, mean ± SD) | 129 ± 36 | 126 ± 33 | 131 ± 38 | 0.31 |
| Time from MI (months; median, quartiles) | 76 (21–137) | 65 (21–126) | 84 (20–155) | 0.29 |
| Male gender (n, %) | 237; 88 | 111; 90 | 126; 88 | 0.38 |
| NYHA | ||||
| I (n, %) | 19; 7 | 14; 11 | 5; 4 | <0.0005 |
| II (n, %) | 132; 50 | 71; 58 | 61; 43 | |
| III (n, %) | 115; 43 | 38; 31 | 77; 54 | |
| Previous revascularization (n; %) | 171; 65 | 81; 68 | 90; 63 | 0.29 |
| Diabetes (n, %) | 81; 30 | 43; 35 | 38; 27 | 0.089 |
| IVCD | ||||
| LBBB (n, %) | 104; 42 | 51; 44 | 53; 41 | 0.745 |
| RBBB (n, %) | 17; 7 | 9; 8 | 8; 6 | |
| None (n, %) | 125; 51 | 56; 48 | 69; 53 | |
| Variable . | All patients (n = 276) . | C-reactive protein ≤3 (n = 124) . | C-reactive protein >3 (n = 144) . | P-value . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 67 ± 10 | 67 ± 10 | 68 ± 10 | 0.17 |
| LVEF (%, mean ± SD) | 26 ± 4 | 26 ± 4 | 26 ± 5 | 0.54 |
| Systolic blood pressure (mmHg; mean ± SD) | 120 ± 18 | 118 ± 17 | 122 ± 18 | 0.12 |
| Diastolic blood pressure (mmHg; mean ± SD) | 75 ± 10 | 74 ± 9 | 75 ± 10 | 0.13 |
| Resting heart rate (b.p.m., mean ± SD) | 72 ± 14 | 69 ± 12 | 74 ± 16 | 0.005 |
| QRS width (ms, mean ± SD) | 129 ± 36 | 126 ± 33 | 131 ± 38 | 0.31 |
| Time from MI (months; median, quartiles) | 76 (21–137) | 65 (21–126) | 84 (20–155) | 0.29 |
| Male gender (n, %) | 237; 88 | 111; 90 | 126; 88 | 0.38 |
| NYHA | ||||
| I (n, %) | 19; 7 | 14; 11 | 5; 4 | <0.0005 |
| II (n, %) | 132; 50 | 71; 58 | 61; 43 | |
| III (n, %) | 115; 43 | 38; 31 | 77; 54 | |
| Previous revascularization (n; %) | 171; 65 | 81; 68 | 90; 63 | 0.29 |
| Diabetes (n, %) | 81; 30 | 43; 35 | 38; 27 | 0.089 |
| IVCD | ||||
| LBBB (n, %) | 104; 42 | 51; 44 | 53; 41 | 0.745 |
| RBBB (n, %) | 17; 7 | 9; 8 | 8; 6 | |
| None (n, %) | 125; 51 | 56; 48 | 69; 53 | |
LVEF, left ventricular ejection fraction; n, number of subjects; NYHA, New York Heart Association; IVCD, intra ventricular conduction delay; LBBB, left bundle branch block; RBBB, right bundle branch block.
Baseline demographics for patients enrolled in the study, overall and per C-reactive protein levels ≤3/>3
| Variable . | All patients (n = 276) . | C-reactive protein ≤3 (n = 124) . | C-reactive protein >3 (n = 144) . | P-value . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 67 ± 10 | 67 ± 10 | 68 ± 10 | 0.17 |
| LVEF (%, mean ± SD) | 26 ± 4 | 26 ± 4 | 26 ± 5 | 0.54 |
| Systolic blood pressure (mmHg; mean ± SD) | 120 ± 18 | 118 ± 17 | 122 ± 18 | 0.12 |
| Diastolic blood pressure (mmHg; mean ± SD) | 75 ± 10 | 74 ± 9 | 75 ± 10 | 0.13 |
| Resting heart rate (b.p.m., mean ± SD) | 72 ± 14 | 69 ± 12 | 74 ± 16 | 0.005 |
| QRS width (ms, mean ± SD) | 129 ± 36 | 126 ± 33 | 131 ± 38 | 0.31 |
| Time from MI (months; median, quartiles) | 76 (21–137) | 65 (21–126) | 84 (20–155) | 0.29 |
| Male gender (n, %) | 237; 88 | 111; 90 | 126; 88 | 0.38 |
| NYHA | ||||
| I (n, %) | 19; 7 | 14; 11 | 5; 4 | <0.0005 |
| II (n, %) | 132; 50 | 71; 58 | 61; 43 | |
| III (n, %) | 115; 43 | 38; 31 | 77; 54 | |
| Previous revascularization (n; %) | 171; 65 | 81; 68 | 90; 63 | 0.29 |
| Diabetes (n, %) | 81; 30 | 43; 35 | 38; 27 | 0.089 |
| IVCD | ||||
| LBBB (n, %) | 104; 42 | 51; 44 | 53; 41 | 0.745 |
| RBBB (n, %) | 17; 7 | 9; 8 | 8; 6 | |
| None (n, %) | 125; 51 | 56; 48 | 69; 53 | |
| Variable . | All patients (n = 276) . | C-reactive protein ≤3 (n = 124) . | C-reactive protein >3 (n = 144) . | P-value . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 67 ± 10 | 67 ± 10 | 68 ± 10 | 0.17 |
| LVEF (%, mean ± SD) | 26 ± 4 | 26 ± 4 | 26 ± 5 | 0.54 |
| Systolic blood pressure (mmHg; mean ± SD) | 120 ± 18 | 118 ± 17 | 122 ± 18 | 0.12 |
| Diastolic blood pressure (mmHg; mean ± SD) | 75 ± 10 | 74 ± 9 | 75 ± 10 | 0.13 |
| Resting heart rate (b.p.m., mean ± SD) | 72 ± 14 | 69 ± 12 | 74 ± 16 | 0.005 |
| QRS width (ms, mean ± SD) | 129 ± 36 | 126 ± 33 | 131 ± 38 | 0.31 |
| Time from MI (months; median, quartiles) | 76 (21–137) | 65 (21–126) | 84 (20–155) | 0.29 |
| Male gender (n, %) | 237; 88 | 111; 90 | 126; 88 | 0.38 |
| NYHA | ||||
| I (n, %) | 19; 7 | 14; 11 | 5; 4 | <0.0005 |
| II (n, %) | 132; 50 | 71; 58 | 61; 43 | |
| III (n, %) | 115; 43 | 38; 31 | 77; 54 | |
| Previous revascularization (n; %) | 171; 65 | 81; 68 | 90; 63 | 0.29 |
| Diabetes (n, %) | 81; 30 | 43; 35 | 38; 27 | 0.089 |
| IVCD | ||||
| LBBB (n, %) | 104; 42 | 51; 44 | 53; 41 | 0.745 |
| RBBB (n, %) | 17; 7 | 9; 8 | 8; 6 | |
| None (n, %) | 125; 51 | 56; 48 | 69; 53 | |
LVEF, left ventricular ejection fraction; n, number of subjects; NYHA, New York Heart Association; IVCD, intra ventricular conduction delay; LBBB, left bundle branch block; RBBB, right bundle branch block.
Events during follow-up and C-reactive protein
Patients were followed for a median of 24 months. During the course of the study 15 patients discontinued the follow-up or withdrew from the study. Two patients who withdrew at the enrolment time were not considered for survival analysis.
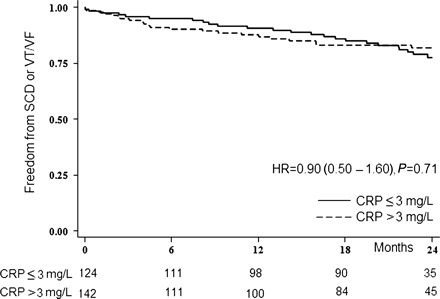
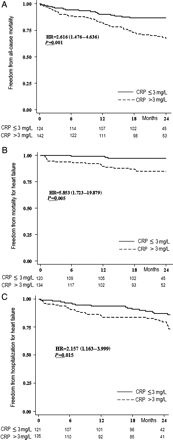
Sixty patients (22.6%) died during the follow-up period: the primary endpoint was reached in 46 patients (17.3%), including 10 (3.8%) with SCD and 36 (13.5%), with fast VT/VF. Twenty-one patients (8.3%) died due to HF-related causes; overall, 42 (15.8%) patients died for cardiac causes and 18 for non-cardiac causes (6.8%). No association between C-reactive protein levels (>3 vs. ≤3 mg/L) and the primary endpoint was found following univariate analysis [HR = 0.897 (0.503–1.599) Table 2]. Correspondingly, the associated Kaplan–Meier curves were almost superimposed (Figure 1). Consistent results were obtained when considering separately each component of the primary endpoint. Similarly, no association with the primary endpoint was evident neither when C-reactive protein was categorized into quartiles nor when it was assessed on a continuous scale (after log-transformation). C-reactive protein levels were associated with all-cause mortality, death due to HF and first hospitalization for HF (Figure 2). The multivariate analysis for competing risks confirmed that the risk of sudden death or ventricular arrhythmias associated with C-reactive protein was close to the null value, while C-reactive protein had a strong association with HF-related mortality (Table 3). Patients with C-reactive protein >3mg/L had a greater than three-fold increase in risk of dying from HF than those with C-reactive protein ≤3 mg/L; this risk remained essentially unchanged after adjusting for covariates in a multivariable analysis [HR: 3.09 (1.37–6.97); P = 0.006).
Univariable association of PCR with composite endpoint of sudden cardiac death or fast ventricular tachycardia/ventricular fibrillation
| Univariable . | |||
|---|---|---|---|
| . | HR (95% CI)a . | P-value . | |
| C-reactive protein >3 | 0.897 | 0.503–1.599 | 0.712 |
| C-reactive protein (IIQ vs. IQ) | 1.120 | 0.503–2.493 | 0.781 |
| C-reactive protein (IIIQ vs. IQ) | 0.92 | 0.284–1.962 | 0.666 |
| C-reactive protein (IVQ vs. IQ) | 1.42 | 0.652–3.138 | 0.373 |
| C-reactive protein (continuous) | 1.145 | 0.905–1.449 | 0.258 |
| Univariable . | |||
|---|---|---|---|
| . | HR (95% CI)a . | P-value . | |
| C-reactive protein >3 | 0.897 | 0.503–1.599 | 0.712 |
| C-reactive protein (IIQ vs. IQ) | 1.120 | 0.503–2.493 | 0.781 |
| C-reactive protein (IIIQ vs. IQ) | 0.92 | 0.284–1.962 | 0.666 |
| C-reactive protein (IVQ vs. IQ) | 1.42 | 0.652–3.138 | 0.373 |
| C-reactive protein (continuous) | 1.145 | 0.905–1.449 | 0.258 |
IQ, quartiles of distribution.
aHazard ratio with 95% confidence interval.
Univariable association of PCR with composite endpoint of sudden cardiac death or fast ventricular tachycardia/ventricular fibrillation
| Univariable . | |||
|---|---|---|---|
| . | HR (95% CI)a . | P-value . | |
| C-reactive protein >3 | 0.897 | 0.503–1.599 | 0.712 |
| C-reactive protein (IIQ vs. IQ) | 1.120 | 0.503–2.493 | 0.781 |
| C-reactive protein (IIIQ vs. IQ) | 0.92 | 0.284–1.962 | 0.666 |
| C-reactive protein (IVQ vs. IQ) | 1.42 | 0.652–3.138 | 0.373 |
| C-reactive protein (continuous) | 1.145 | 0.905–1.449 | 0.258 |
| Univariable . | |||
|---|---|---|---|
| . | HR (95% CI)a . | P-value . | |
| C-reactive protein >3 | 0.897 | 0.503–1.599 | 0.712 |
| C-reactive protein (IIQ vs. IQ) | 1.120 | 0.503–2.493 | 0.781 |
| C-reactive protein (IIIQ vs. IQ) | 0.92 | 0.284–1.962 | 0.666 |
| C-reactive protein (IVQ vs. IQ) | 1.42 | 0.652–3.138 | 0.373 |
| C-reactive protein (continuous) | 1.145 | 0.905–1.449 | 0.258 |
IQ, quartiles of distribution.
aHazard ratio with 95% confidence interval.
Competing risk multivariable analysis for different components of mortality: primary endpoint (sudden cardiac death or ventricular fibrillation or fast ventricular tachycardia); death for heart failure, death for other causes, according to C-reactive protein >3 vs. C-reactive protein ≤3
| . | HR (95% CI)a . | P-value . | |
|---|---|---|---|
| C-reactive protein—sudden cardiac death or VF or fast VT | 0.91 | 0.49–1.69 | 0.76 |
| C-reactive protein—death for heart failure | 3.09 | 1.37–6.97 | 0.006 |
| C-reactive protein—death for other causes | 1.79 | 0.46–6.96 | 0.40 |
| . | HR (95% CI)a . | P-value . | |
|---|---|---|---|
| C-reactive protein—sudden cardiac death or VF or fast VT | 0.91 | 0.49–1.69 | 0.76 |
| C-reactive protein—death for heart failure | 3.09 | 1.37–6.97 | 0.006 |
| C-reactive protein—death for other causes | 1.79 | 0.46–6.96 | 0.40 |
aHazard ratio with 95% confidence interval (estimates adjusted for Cystatin-C, NTproBNP, LVEF, heart rate, NYHA, QRS width, diabetes, previous revascularization, dilated post-ischaemic aetiology, peripheral artery disease, history of stroke, and current smoker).
Competing risk multivariable analysis for different components of mortality: primary endpoint (sudden cardiac death or ventricular fibrillation or fast ventricular tachycardia); death for heart failure, death for other causes, according to C-reactive protein >3 vs. C-reactive protein ≤3
| . | HR (95% CI)a . | P-value . | |
|---|---|---|---|
| C-reactive protein—sudden cardiac death or VF or fast VT | 0.91 | 0.49–1.69 | 0.76 |
| C-reactive protein—death for heart failure | 3.09 | 1.37–6.97 | 0.006 |
| C-reactive protein—death for other causes | 1.79 | 0.46–6.96 | 0.40 |
| . | HR (95% CI)a . | P-value . | |
|---|---|---|---|
| C-reactive protein—sudden cardiac death or VF or fast VT | 0.91 | 0.49–1.69 | 0.76 |
| C-reactive protein—death for heart failure | 3.09 | 1.37–6.97 | 0.006 |
| C-reactive protein—death for other causes | 1.79 | 0.46–6.96 | 0.40 |
aHazard ratio with 95% confidence interval (estimates adjusted for Cystatin-C, NTproBNP, LVEF, heart rate, NYHA, QRS width, diabetes, previous revascularization, dilated post-ischaemic aetiology, peripheral artery disease, history of stroke, and current smoker).
Kaplan–Meier plot of freedom from sudden cardiac death or ventricular tachycardia/ventricular fibrillation during follow-up according to C-reactive protein >3 vs. C-reactive protein ≤3. CRP, C-reactive protein; HR, hazard ratio.
Kaplan–Meier plot of freedom from (A) all-cause mortality (B) mortality for heart failure (C) hospitalization for heart failure, during follow-up according to C-reactive protein >3 vs. C-reactive protein ≤3. CRP, C-reactive protein; HR, hazard ratio.
Multi-marker analysis
NT-proBNP value above the median was significantly associated with the composite primary endpoint [adjusted HR: 1.46 (1.020–2.129); P = 0.042] and with occurrence of death from any cause [adjusted HR: 5.25 (2.724–10–142); P < 0.001). Cystatin-C above the median was not associated with the primary endpoint [adjusted HR: 1.57 (0.871–2.834); P = 0.133] but was associated with all-cause mortality [adjusted HR: 2.61 (1.502–4.533); P = 0.001].
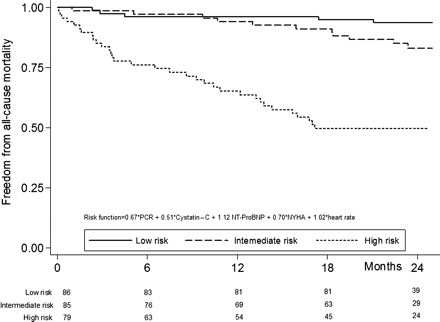
When including the three biomarkers and the baseline clinical variables in a multivariable model, in order to assess their independent prognostic role on all-cause mortality, both C-reactive protein (>3 mg/L) and NT-proBNP (above median) together with NYHA class >II and HR >70 (among clinical parameters) showed a significant association with mortality (Table 4), C-reactive protein (above median) though marginally non-significant, and associated with a 1.7-fold increase in risk. Cystatin-C was removed from the model. The risk function for all-cause mortality including those biochemical and clinical variables, when divided in tertiles, showed a high discriminating power (Harrell's c = 0.78), eliciting a 2-year morality rate below of 5% in the low risk group vs. a value equal to 50% in the high risk group (Figure 3). Moreover, if biomarkers were excluded from the model, discrimination dropped (c-statistic = 0.72).
Association of serum biomarkers and clinical variables with all-cause mortality
| Overall association: χ2 = 57.3, P < 0.001 Harrel's c = 0.77 . | |||
|---|---|---|---|
| Variable . | HR (95% CI)a . | P-value . | |
| C-reactive protein | 1.95 | 1. 06–3.56 | 0.031 |
| Cystatin-C | 1.67 | 0.91–3.05 | 0.095 |
| NT Pro-BNP | 3.07 | 1.–6.56 | 0.004 |
| NYHA class >II | 2.01 | 1.12–3.59 | 0.019 |
| Resting heart rate >70 | 2.78 | 1.48–5.01 | 0.001 |
| Overall association: χ2 = 57.3, P < 0.001 Harrel's c = 0.77 . | |||
|---|---|---|---|
| Variable . | HR (95% CI)a . | P-value . | |
| C-reactive protein | 1.95 | 1. 06–3.56 | 0.031 |
| Cystatin-C | 1.67 | 0.91–3.05 | 0.095 |
| NT Pro-BNP | 3.07 | 1.–6.56 | 0.004 |
| NYHA class >II | 2.01 | 1.12–3.59 | 0.019 |
| Resting heart rate >70 | 2.78 | 1.48–5.01 | 0.001 |
aHazard ratio with 95% confidence interval.
Backward stepwise selection original variables: C-reactive protein, Cystatin-C, NT-Pro BNP, LVEF, resting heart rate, NYHA class, QRS duration, diabetes, previous revascularization, dilated post-ischaemic aetiology, peripheral artery disease, history of stroke, and current smoker. Removed variables with P≥ 0.1: diabetes, LVEF, QRS duration, previous revascularization, NYHA class, cystatin-C, peripheral artery disease, current smoker.
NYHA, New York Heart Association.
C-reactive protein = 1 if >3 mg/L; cystatin-C = 1 if >1.25 mg/L; NT-Pro BNP = 1 if >1610 pg/mL.
Association of serum biomarkers and clinical variables with all-cause mortality
| Overall association: χ2 = 57.3, P < 0.001 Harrel's c = 0.77 . | |||
|---|---|---|---|
| Variable . | HR (95% CI)a . | P-value . | |
| C-reactive protein | 1.95 | 1. 06–3.56 | 0.031 |
| Cystatin-C | 1.67 | 0.91–3.05 | 0.095 |
| NT Pro-BNP | 3.07 | 1.–6.56 | 0.004 |
| NYHA class >II | 2.01 | 1.12–3.59 | 0.019 |
| Resting heart rate >70 | 2.78 | 1.48–5.01 | 0.001 |
| Overall association: χ2 = 57.3, P < 0.001 Harrel's c = 0.77 . | |||
|---|---|---|---|
| Variable . | HR (95% CI)a . | P-value . | |
| C-reactive protein | 1.95 | 1. 06–3.56 | 0.031 |
| Cystatin-C | 1.67 | 0.91–3.05 | 0.095 |
| NT Pro-BNP | 3.07 | 1.–6.56 | 0.004 |
| NYHA class >II | 2.01 | 1.12–3.59 | 0.019 |
| Resting heart rate >70 | 2.78 | 1.48–5.01 | 0.001 |
aHazard ratio with 95% confidence interval.
Backward stepwise selection original variables: C-reactive protein, Cystatin-C, NT-Pro BNP, LVEF, resting heart rate, NYHA class, QRS duration, diabetes, previous revascularization, dilated post-ischaemic aetiology, peripheral artery disease, history of stroke, and current smoker. Removed variables with P≥ 0.1: diabetes, LVEF, QRS duration, previous revascularization, NYHA class, cystatin-C, peripheral artery disease, current smoker.
NYHA, New York Heart Association.
C-reactive protein = 1 if >3 mg/L; cystatin-C = 1 if >1.25 mg/L; NT-Pro BNP = 1 if >1610 pg/mL.
Kaplan–Meier survival curves for all-cause mortality according to the tertiles of the risk function, calculated from the multivariable model. The upper tertile represents high-risk patients having increased, CRP, NT-Pro BNP, dilated post-ischaemic aetiology, previous stroke, and higher resting HR. BNP, brain natriuretic peptide; HR, heart rate; CRP, C-reactive protein; NYHA, New York Heart Association.
Discussion
Despite several trials demonstrating the efficacy of ICDs in reducing sudden and all-cause mortality in ischaemic and non-ischaemic patients1–6 and the fact that international guidelines have been updated accordingly, ICDs are still underused in clinical practice among different geographies.7–9 Implantable cardioverter defibrillator eligibility is commonly debated, particularly when only LVEF is used in the selection of ICD candidates. The limited sensitivity of the LVEF to predict risk of sudden death, as stressed by Buxton et al.,13 may be due to the complex and, probably, incompletely understood pathophysiology of life-threatening arrhythmias. Presently, there is still a lack of validated criteria for additional selection for prophylactic ICD therapy among patients with reduced LVEF; the recent focus on both clinical and non-invasive ECG risk predictors such as QRS duration, T-waves alternans, HR turbulence, and QT variability failed to produce clinically relevant information to date.15,25,26
In this study, we tested the hypothesis that biological markers associated with acute ischaemia or plaque instability may be associated with the mechanisms of SCD or life-threatening ventricular arrhythmias. We focused primarily on high sensitivity C-reactive protein, and, additionally, on a multi-marker approach to investigate risk stratification in post-MI patients with depressed ventricular function.
C-reactive protein, an acute-phase protein associated with inflammatory disorders, has been significantly correlated in ischaemic patients with infarct extension, complications and poor outcome.27,28 Elevated levels of C-reactive protein have been demonstrated to be highly predictive of the risk of death in patients with ischaemic heart disease29–31 and in apparently healthy subjects.32 In particular, elevated C-reactive protein levels turned out to be associated with sudden coronary death due to plaque rupture and with the risk of future sudden death in healthy subjects.29,30 The CAMI-GUIDE study for the first time tested prospectively the hypothesis that high sensitivity C-reactive protein may play a role in sudden death in patients with previous MI and severely depressed LVEF and may represent a guide for additional risk stratification in patients indicated for ICD implantation according to current guidelines. This study failed to demonstrate an association between C-reactive protein, either on a discrete (quartiles, binomial) or continuous scale, and the occurrence of a composite endpoint of SCD or threatening ventricular arrhythmias and, therefore, to validate C-reactive protein as a marker of risk in these patients. With a hazard ratio close to 1, this study provides no evidence of possible associations between C-reactive protein levels before ICD implantation and future appropriate therapy delivery or SCD. However, the clinical design of the CAMI-GUIDE study, with C-reactive protein levels assessed at ICD implantation and events occurring up to 2 years post-implant, does not allow us to completely exclude the hypothesis that a rise in C-reactive protein during follow-up might have anticipated the occurrence of life-threatening arrhythmias in our population. Therefore, we cannot completely rule out a role of C-reactive protein and inflammation in SCD of ischaemic patients.
The absence of any association between C-reactive protein and ventricular arrhythmias in this sample, apparently in contrast with other results obtained in previous studies,33–36 may be explained by differences in the enrolled population. C-reactive protein has been shown to be elevated in subjects studied at autopsy after sudden coronary death in association with plaque rupture29 and in healthy subjects at risk of future sudden death;30 however, these studies included populations with a low cardiovascular risk. Conversely, the CAMI-GUIDE study involved patients with a higher risk profile, having previous infarction, low ejection fraction and, in several cases, HF. It might be therefore possible that C-reactive protein may emerge as a marker of SCD in low risk population, but that the risk conferred by C-reactive protein is completely blunted in the presence of stronger factors as those present in our population. Additionally, the mechanisms associated to SCD and ventricular arrhythmias may be different: Burke29 found high levels of C-reactive protein in the blood and plaque of patients died suddenly for a coronary plaque rupture, while in our study the occurrence of new ischaemic events was definitely low. This suggests that plaque rupture and acute ischaemia, conditions that C-reactive protein may be able to detect, were not the major cause of VT/VF occurrence in our patients and that re-entry mechanism was dominant cause for the events. Although this conclusion may appear obvious, it is an important confirmatory observation, as several authors have stressed the possibility that different ischaemia-associated electrophysiological mechanisms may induce TV and FV.16–19 To increase sensitivity of our analysis for ischaemic life threatening arrhythmias, we had selected as endpoints FV and fast VT, i.e. those with a HR >200, that are supposed to have a significant ischaemic component. The results from the CAMI-GUIDE study outline the complexity in matter of predicting major ventricular arrhythmias, probably reflecting a poor understanding of their mechanisms in this population, where left ventricular dysfunction, myocardial ischaemia, scar, and sympathetic hyperactivity may all play a different role in each patient.
Conversely, a more recent analysis of the MADIT II study analysing 8 year outcomes,37 showed that ICD implantation based exclusively on low ejection fraction is more efficient in terms of patients needed to treat to save one life than originally thought. Therefore debates for additional risk stratification could be mitigated when taking into account the long-term outcomes of these patients and published guidelines recommending ICD implantation for patients with low ejection fraction could be reinforced.
On the other hand, in this study C-reactive protein was found to be a strong predictor of death for HF and of re-hospitalization for HF, endpoints that have been previously analysed retrospectively or in single-centre studies.38–40 The observation that C-reactive protein levels predict HF mortality and re-hospitalization is intriguing: despite resulting from a secondary analysis, this finding suggests a role of inflammation in the pathophysiology of HF. Additionally, this result supports the use of C-reactive protein as a marker of HF risk and underlines the potential adjunctive role of a CRT implant in post-MI patients candidates for an ICD with elevated C-reactive protein, in order to prevent disease progression. As a matter of fact, more recently, the MADIT-CRT41 and REVERSE42 trials demonstrated the effectiveness of CRT in those patients who were previously indicated only for an ICD.
Multiple biomarkers assessment
NT-proBNP and Cystatin-C were measured to evaluate the potential additive benefit of a multi-marker approach for risk stratification in these patients. Cystatin-C is a marker of renal function, more accurate than creatinine and glomerular filtration rate, which is an excellent marker of death in ischaemic patients.43,44 NT-proBNP is the amino terminal part of BNP, a natriuretic peptide that has been consistently shown to be an excellent marker of HF and, in small, recent studies, of sudden death/VT/VF in ICD recipients.45–48
Results of this further analysis provide evidence that NT-proBNP is not only a significant predictor of death for HF, but it is also a predictor of VT/VF and sudden death. The complex pathogenesis of the major ventricular arrhythmias in high-risk post-MI patients might explain why NT-proBNP, which is an accurate marker of LV dysfunction, neuro-hormonal activation, and inflammation, is a predictor of life-threatening arrhythmias and sudden death.45 The relatively short duration of follow-up might explain why renal function, assessed by Cystatin-C levels, does not predict the outcome in this study (although an almost two-fold increase in risk of dying from any cause was shown). The independent association of biomarkers with all-cause mortality, together with resting HR >70 b.p.m., dilated post-ischaemic aetiology and previous stroke, led to a further improvement of risk stratification that may be of clinical interest. Indeed, it allows identifying subjects with markedly different risk profiles within an apparently homogeneous population of post-MI patients with depressed ventricular function. These findings outline the multi-facet nature of cardiac death in post-MI patients with low LVEF in whom no single risk factor can cover all clinical and patho-physiological aspects and stress the importance of a multi-marker approach.49,50
The present study has inherent limitations that include the absence of randomization and the fact that the composite endpoint can be considered only a surrogate of SCD, as VT/VF episodes treated by the device cannot be considered equivalent to SCD. However, while closing the doors to pre-implant C-reactive protein as a prognostic marker of sudden death in this population, this study showed that a multi-marker approach, used together with traditional clinical predictors of death (such as HR and NYHA class) results in a better risk stratification of these patients. Therefore, these findings open the possibility to identify subsets with different risk profiles and may help physicians to optimize the management of available resources when treating ischaemic patients indicated for an ICD.
Funding
This work was sponsored by Boston Scientific.
Conflict of interest: C.K. has received consulting fees from Boston Scientific. F.A. is an employee of Boston Scientific.
Acknowledgements
We acknowledge the study Advisory Board members (F.B., L.M.B., G.F. Gensini, L. Padeletti, A. Raviele, M.S.), the Event Adjudication Committee (G.Z., S.O., C. Vasco, R. Luise) the Study Core Lab in Policlinico Agostino Gemelli, Roma, Italy (G.G., N. Vitulano, A. Feminò, M. Narducci) and the study Sponsor (F.A., G.R., L. Sallusti, D. Brandi) for their contribution to this study.