-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-Claude Tardif, Eileen O'Meara, Michel Komajda, Michael Böhm, Jeffrey S. Borer, Ian Ford, Luigi Tavazzi, Karl Swedberg, on behalf of the SHIFT Investigators, Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy, European Heart Journal, Volume 32, Issue 20, October 2011, Pages 2507–2515, https://doi.org/10.1093/eurheartj/ehr311
Close - Share Icon Share
Abstract
The SHIFT echocardiographic substudy evaluated the effects of ivabradine on left ventricular (LV) remodelling in heart failure (HF).
Eligible patients had chronic HF and systolic dysfunction [LV ejection fraction (LVEF) ≤35%], were in sinus rhythm, and had resting heart rate ≥70 bpm. Patients were randomly allocated to ivabradine or placebo, superimposed on background therapy for HF. Complete echocardiographic data at baseline and 8 months were available for 411 patients (ivabradine 208, placebo 203). Treatment with ivabradine reduced LVESVI (primary substudy endpoint) vs. placebo [−7.0 ± 16.3 vs. −0.9 ± 17.1 mL/m2; difference (SE), −5.8 (1.6), 95% CI −8.8 to −2.7, P< 0.001]. The reduction in LVESVI was independent of beta-blocker use, HF aetiology, and baseline LVEF. Ivabradine also improved LV end-diastolic volume index (−7.9 ± 18.9 vs. −1.8 ± 19.0 mL/m2, P= 0.002) and LVEF (+2.4 ± 7.7 vs. −0.1 ± 8.0%, P< 0.001). The incidence of the SHIFT primary composite outcome (cardiovascular mortality or hospitalization for worsening HF) was higher in patients with LVESVI above the median (59 mL/m2) at baseline (HR 1.62, 95% CI 1.03–2.56, P= 0.04). Patients with the largest relative reductions in LVESVI had the lowest event rates.
Ivabradine reverses cardiac remodelling in patients with HF and LV systolic dysfunction.
See page 2481 for the commentary on this article (doi:10.1093/eurheartj/ehr317)
Introduction
Cardiac remodelling is central to the pathophysiology of heart failure (HF)1 and is an established prognostic factor in patients with HF. Left ventricular (LV) enlargement has been shown to be associated with an increased risk for adverse cardiac events,2 while reduced LV ejection fraction (LVEF) is a powerful predictor of cardiovascular outcomes and all-cause mortality.3 The therapeutic effects of beta-blockade, angiotensin-converting enzyme (ACE) inhibition, and cardiac resynchronization therapy have been linked to their beneficial effects on cardiac remodelling.1,4 A recent analysis compared 30 clinical event trials and 88 remodelling trials with the same drug or device therapy and reported that the odds ratio associated with therapy for long-term mortality correlate with the shorter term effect of the therapy on LVEF, end-systolic volume, and end-diastolic volume.5 It is therefore relevant to evaluate the impact of novel HF therapies on cardiac remodelling.
The heart rate lowering effect of ivabradine, a specific inhibitor of the If current in the sinoatrial node, has been found to be beneficial in patients with HF. The results of the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) showed that treatment with ivabradine superimposed on background therapy for HF was associated with an 18% reduction in risk for the primary composite endpoint of cardiovascular death or hospitalization for worsening HF (P< 0.0001).6 In this article, we present the results of the prespecified echocardiographic substudy of the SHIFT trial, aiming to evaluate the effects of ivabradine vs. placebo on LV remodelling and function in HF.
Methods
Study design and patients
The design of the SHIFT study has been described in detail in previous publications.6,7 Briefly, this randomized, double-blind, placebo-controlled, parallel-group, international multicentre clinical trial evaluated 6505 male or female patients with moderate-to-severe chronic HF and documented LV systolic dysfunction (LVEF ≤35%). Eligible patients were in sinus rhythm and had resting heart rate of ≥70 beats per minute (bpm) on 12-lead electrocardiography; they had been clinically stable for ≥4 weeks, and had been admitted to hospital for worsening HF within the previous 12 months. All participants were receiving background therapy for HF, including a beta-blocker. Other inclusion and exclusion criteria are detailed elsewhere.6,7 After a 2-week run-in period, patients were randomly allocated to receive ivabradine 5 mg bid or placebo. Randomization was stratified according to beta-blocker intake at randomization and country. At subsequent visits at 14 days, 28 days, and every 4 months thereafter (or at any other time if necessary), the dosage of blinded study medication could be adjusted upward (7.5 mg bid) or downward (2.5 mg bid) depending on resting heart rate and tolerability.
The SHIFT echocardiography substudy was performed in 89 centres in 21 countries participating in the main SHIFT study. To avoid selection bias, all the patients in each participating centre were invited to enter the substudy, and those included signed a specific informed consent form in addition to the documents related to the main study. The locally appointed Ethics Committees have approved the research protocol. The substudy was carried out in accordance with the Declaration of Helsinki, 1964, and its text revisions. The SHIFT trial is registered, number ISRCTN70429960.
The primary endpoint of the SHIFT echocardiography substudy was the change in the LV end-systolic volume index (LVESVI) from baseline to 8 months. Secondary endpoints were changes in LV end-diastolic volume index (LVEDVI), non-indexed LV end-systolic and end-diastolic volumes (LVESV and LVEDV), and LVEF during the same interval. Tertiary endpoints included left atrial end-systolic volume index, grade of diastolic dysfunction (assessed by Doppler imaging), grade of mitral regurgitation, right ventricular (RV) myocardial performance index, and RV s wave peak velocity (assessed by Doppler imaging). The 8-month duration of the substudy was selected because it was considered long enough to allow detection of a benefit on LV remodelling on the basis of the observed effects of ACE inhibitors and beta-blockers1 while minimizing loss of patients to follow-up because of death in this HF population.
Echocardiographic imaging and analyses
Echocardiography was performed at baseline (in the 2 weeks between selection and inclusion) and within 1 month of the 8-month visit. The two recordings were to be made by the same technician using the same technique and the same equipment. Transthoracic echocardiography was performed with a phased-array imaging system equipped with a transducer with second harmonics capability. Images were obtained in the parasternal long- and short-axis and apical views, with the subject lying in the left lateral position. Tissue Doppler imaging was performed in the centres where technically feasible. All images were stored digitally on CD-ROM. All participating sites received a detailed imaging manual before initiation of the substudy, needed to be certified by the core laboratory, and were regularly monitored for and informed of quality of imaging recordings throughout the study.
The echocardiograms were read centrally according to predefined procedures in the core laboratory at the Montreal Heart Institute (Canada) by technicians supervised by two cardiologists, all of them blinded to treatment and examination sequence (baseline vs. follow-up). Both recordings from the same patient were assessed during two separate imaging analysis sessions by the same technician and validated by the same cardiologist, without knowledge of the results of the other imaging exam (i.e. results of the first recording analysis were not known to the core laboratory team when the second recording from the same patient was analysed). All measurements were made according to the recommendations of the American Society of Echocardiography (ASE) and the European Association of Echocardiography.8 Each assessment was analysed for at least three consecutive cycles (or at least five in case of atrial fibrillation), avoiding post-ectopic beats. Atrial fibrillation was an exclusion criterion in the SHIFT study. Two patients in the ivabradine group had atrial fibrillation at the time of the 8-month echocardiogram; four patients (two ivabradine, two placebo) sustained atrial fibrillation during the substudy (M0 to M8). Primary and secondary criteria were assessed on two-dimensional apical four- and two-chamber views, using biplane views when they were analysable at both time points; if biplane apical views were not available at baseline and follow-up, the analysis was conducted using the apical four-chamber view. Patients who only had analysable images from the apical two-chamber view were excluded from the substudy. Each recording was graded for the quality of LV endocardial border resolution: Grade 1, acceptable four- and two-chamber views; Grade 2, acceptable four- or two-chamber view; Grade 3, poor quality four- and two-chamber views. Grade 3 recordings were excluded from the analysis of LV volumes. Grade 3 was assigned to recordings with insufficient resolution of LV endocardial border in 4 or more of the 17 ventricular segments (using the ASE 17-segment model). Inter-reader and intra-reader variability was evaluated at the echocardiography core laboratory using 60 recordings analysed twice by each technician. Intra-class correlation coefficients (ICCs) were found to be satisfactory for all readers (ranges for LVESVI: intra-reader ICC, 0.96–0.99; inter-reader ICC, 0.95–0.98). Left ventricular diastolic function was classified as normal, impaired LV relaxation, pseudonormal, or restrictive based on standard criteria.
Statistical analysis
The sample size was estimated to demonstrate the superiority of ivabradine over placebo on the change from baseline to 8 months in LVESVI (primary substudy endpoint), using a two-sided Student's t-test for independent samples at a 5% type I error rate. Considering a standard deviation of 25 mL/m2, it was estimated that 414 patients would be necessary to detect at least 8 mL/m2 difference between treatment groups with 90% power. The sample size was adjusted to 520 patients since it was anticipated that the change from baseline in LVESVI would not be assessable for approximately 20% of patients, on the basis of the estimated rate of mortality and the projected proportion of non-assessable echocardiographic recordings.
Baseline characteristics were presented by treatment group on all patients randomized in the main study and participating in the echocardiography substudy, with means ± SD for continuous variables, and numbers and percentages for categorical variables. Changes in primary and secondary endpoints were compared between treatment groups on patients included in the substudy who had taken at least one dose of study drug and with an evaluation of the primary endpoint considered reliable, using a covariance analysis adjusted for beta-blocker intake at randomization, country, and baseline value. The treatment effect was estimated using adjusted least square means from this model, with associated two-sided 95% confidence intervals (CI) and P-values (significance level set at 5%). A sensitivity analysis with imputation for missing values of LVESVI (imputing conservatively no change in the ivabradine arm and a mean change from baseline of −10 mL/m2 in the placebo arm) indicated that the results are not unduly influenced by missing data. Changes in LVESVI and LVEF from baseline to 8 months were divided into three classes (improvement, no change, and worsening) according to clinical thresholds (15% for LVESVI and 5% for LVEF, respectively). The percentages of patients with an improvement were compared between treatment groups using a χ2 test. The relationship between baseline echocardiography criteria and the SHIFT primary composite endpoint (defined as first event among hospitalization for worsening HF or cardiovascular death) as well as its components was investigated in patients included in the substudy and allocated to the placebo group. Cox proportional hazards models adjusted for the echocardiography endpoint as a dichotomous variable (below and above the median value) and for other relevant factors at baseline [age, New York Heart Association (NYHA) class, heart rate, ischaemic aetiology, systolic blood pressure, estimated glomerular filtration rate, and beta-blocker intake] were used to estimate hazard ratio comparing classes of baseline echocardiography endpoints, with associated 95% CIs and P-values. Time-to-event curves were presented by class of baseline LVESVI and estimated using the Kaplan–Meier method. The number and percentage of SHIFT primary composite endpoints subsequent to 8 months was calculated in each group divided by tertile of relative change in LVESVI from baseline to 8 months, for ivabradine and placebo groups separately. The correlation between changes in LVEF and heart rate was tested using the linear regression model including the two treatment groups.
The analyses on primary and secondary endpoints of the substudy were all prespecified (LVESVI, LVEDVI, LVESV, LVEDV, and LVEF), as was the case for tertiary echocardiographic endpoints (e.g. mitral regurgitation). The exploratory analyses linking echocardiographic parameters to outcomes or heart rate were performed on a post hoc basis. SAS (version 9.1) was used for all analyses.
Role of the funding source
The sponsor was responsible for data management and final data analyses. The independent centre for statistics Keyrus Biopharma (Nantes, France) verified all analyses. The cardiologists in charge of the core laboratory and the SHIFT executive committee were responsible for the study design, the interpretation of the results, the development and writing of the report, and the decision to submit for publication and had full access to all data. Members of the medical and scientific departments of the sponsor supported the work of the executive committee, but did not make any scientific or research decisions independent of this committee.
Results
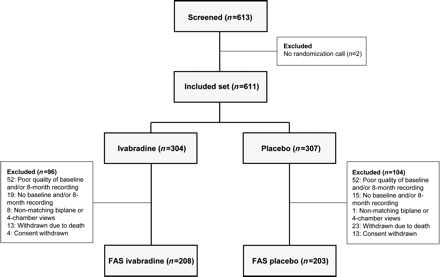
A total of 613 patients were screened for the substudy and 611 were included (304 ivabradine, 307 placebo, Figure 1). A total of 200 patients were excluded leaving 411 patients (67% of the included set, 208 ivabradine and 203 placebo) for analysis. The main reasons for exclusion were poor quality of two- or four-chamber views (104 patients with insufficient resolution of LV endocardial border, precluding reliable measurement of LV volumes), no baseline and/or 8-month recording received at the core laboratory (34 patients), and no matching apical biplane or apical four-chamber views (nine patients). Other reasons for exclusion were death (36 patients) and consent withdrawal (17 patients).
The characteristics of the substudy population at baseline are presented in Table 1, and compared with those of the main study population.6 The mean age of the population was 59.7 years. The majority of patients were men (81%) and Caucasians (91%). Mean resting heart rate at baseline was 78.6 ± 9.1 bpm and 66% had HF of ischaemic aetiology. The population was evenly distributed between NYHA classes II and III. Most of the patients were receiving a beta-blocker (92%) and a renin–angiotensin–aldosterone system (RAAS) antagonist (94%). The main beta-blockers used were carvedilol, bisoprolol, and metoprolol succinate (Table 2), with 29% of the population at guidelines-based target dose of beta-blocker and 56% receiving ≥50% of the target dose. The characteristics of the substudy population were similar to those of the population in the main study, except for a lower rate of history of hypertension in the substudy (59 vs. 66%) and a higher rate of use of aldosterone antagonists (72 vs. 60%). There were no clinically relevant differences between the treatment groups in the substudy, between the SHIFT patients included in the substudy and SHIFT patients not included in the substudy (see online Supplementary Data), or between patients randomized in the study and the full analysis set for the substudy.
Baseline characteristics of the substudy population (included set) vs. the population of the main SHIFT study6 (randomized set)
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| Demographic parameters | ||||
| Age (years) | 60.4 ± 10.9 | 59.1 ± 11.1 | 59.7 ± 11.0 | 60.4 ± 11.4 |
| Sex (male) | 244 (80%) | 252 (82%) | 496 (81%) | 4970 (76%) |
| Caucasian | 276 (91%) | 281 (92%) | 557 (91%) | 5771 (89%) |
| Current smoker | 48 (16%) | 48 (16%) | 96 (16%) | 1118 (17%) |
| BMI (kg/m2) | 27.7 ± 4.5 | 27.7 ± 4.9 | 27.7 ± 4.7 | 28.0 ± 5.1 |
| Cardiac parameters | ||||
| Heart rate (bpm) | 78.4 ± 9.0 | 78.8 ± 9.2 | 78.6 ± 9.1 | 79.9 ± 9.6 |
| Heart rate ≥77 bpm | 137 (45%) | 129 (42%) | 266 (44%) | 3357 (52%) |
| Sitting SBP (mm Hg) | 120.5 ± 15.9 | 119.1 ± 15.4 | 119.8 ± 15.6 | 121.7 ± 16.0 |
| Sitting DBP (mm Hg) | 75.2 ± 9.3 | 75.4 ± 9.0 | 75.3 ± 9.1 | 75.7 ± 9.5 |
| eGFR (MDRD formula) (mL/min/1.73 m2) | 73.7 ± 20.6 | 75.8 ± 25.3 | 74.7 ± 23.1 | 74.7 ± 23.0 |
| NYHA class | ||||
| Class II | 146 (48%) | 140 (46%) | 286 (47%) | 3169 (49%) |
| Class III | 156 (51%) | 164 (53%) | 320 (52%) | 3223 (50%) |
| Class IV | 2 (0.7%) | 3 (1.0%) | 5 (0.8%) | 111 (1.7%) |
| Medical history | ||||
| Duration of heart failure (years) | 3.5 ± 4.0 | 3.5 ± 4.5 | 3.5 ± 4.3 | 3.5 ± 4.2 |
| Primary cause of heart failure | ||||
| Ischaemic | 205 (67%) | 200 (65%) | 405 (66%) | 4418 (68%) |
| Non-ischaemic | 99 (33%) | 107 (35%) | 206 (34%) | 2087 (32%) |
| Myocardial infarction | 180 (59%) | 178 (58%) | 358 (59%) | 3666 (56%) |
| Hypertension | 179 (59%) | 175 (57%) | 354 (58%) | 4314 (66%) |
| Diabetes | 96 (32%) | 98 (32%) | 194 (32%) | 1979 (30%) |
| Previous stroke | 25 (8%) | 28 (9%) | 53 (9%) | 522 (8%) |
| History atrial fibrillation and/or atrial flutter | 18 (6%) | 20 (7%) | 38 (6%) | 522 (8%) |
| Left bundle branch block | 45 (15%) | 40 (13%) | 85 (14%) | 865 (13%) |
| Treatment at randomization | ||||
| β-blocker | 281 (92%) | 281 (92%) | 562 (92%) | 5820 (90%) |
| ACE inhibitor | 243 (80%) | 255 (83%) | 498 (82%) | 5116 (79%) |
| ARB | 51 (17%) | 36 (12%) | 87 (14%) | 927 (14%) |
| Diuretic (excluding antialdosterone) | 264 (87%) | 266 (87%) | 530 (87%) | 5414 (83%) |
| Aldosterone antagonist | 224 (74%) | 218 (71%) | 442 (72%) | 3922 (60%) |
| Digitalis | 83 (27%) | 99 (32%) | 182 (30%) | 1416 (22%) |
| Devices | ||||
| At least one device | 10 (3%) | 13 (4%) | 23 (4%) | 244 (4%) |
| ICD | 9 (3%) | 11 (4%) | 20 (3%) | 207 (3%) |
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| Demographic parameters | ||||
| Age (years) | 60.4 ± 10.9 | 59.1 ± 11.1 | 59.7 ± 11.0 | 60.4 ± 11.4 |
| Sex (male) | 244 (80%) | 252 (82%) | 496 (81%) | 4970 (76%) |
| Caucasian | 276 (91%) | 281 (92%) | 557 (91%) | 5771 (89%) |
| Current smoker | 48 (16%) | 48 (16%) | 96 (16%) | 1118 (17%) |
| BMI (kg/m2) | 27.7 ± 4.5 | 27.7 ± 4.9 | 27.7 ± 4.7 | 28.0 ± 5.1 |
| Cardiac parameters | ||||
| Heart rate (bpm) | 78.4 ± 9.0 | 78.8 ± 9.2 | 78.6 ± 9.1 | 79.9 ± 9.6 |
| Heart rate ≥77 bpm | 137 (45%) | 129 (42%) | 266 (44%) | 3357 (52%) |
| Sitting SBP (mm Hg) | 120.5 ± 15.9 | 119.1 ± 15.4 | 119.8 ± 15.6 | 121.7 ± 16.0 |
| Sitting DBP (mm Hg) | 75.2 ± 9.3 | 75.4 ± 9.0 | 75.3 ± 9.1 | 75.7 ± 9.5 |
| eGFR (MDRD formula) (mL/min/1.73 m2) | 73.7 ± 20.6 | 75.8 ± 25.3 | 74.7 ± 23.1 | 74.7 ± 23.0 |
| NYHA class | ||||
| Class II | 146 (48%) | 140 (46%) | 286 (47%) | 3169 (49%) |
| Class III | 156 (51%) | 164 (53%) | 320 (52%) | 3223 (50%) |
| Class IV | 2 (0.7%) | 3 (1.0%) | 5 (0.8%) | 111 (1.7%) |
| Medical history | ||||
| Duration of heart failure (years) | 3.5 ± 4.0 | 3.5 ± 4.5 | 3.5 ± 4.3 | 3.5 ± 4.2 |
| Primary cause of heart failure | ||||
| Ischaemic | 205 (67%) | 200 (65%) | 405 (66%) | 4418 (68%) |
| Non-ischaemic | 99 (33%) | 107 (35%) | 206 (34%) | 2087 (32%) |
| Myocardial infarction | 180 (59%) | 178 (58%) | 358 (59%) | 3666 (56%) |
| Hypertension | 179 (59%) | 175 (57%) | 354 (58%) | 4314 (66%) |
| Diabetes | 96 (32%) | 98 (32%) | 194 (32%) | 1979 (30%) |
| Previous stroke | 25 (8%) | 28 (9%) | 53 (9%) | 522 (8%) |
| History atrial fibrillation and/or atrial flutter | 18 (6%) | 20 (7%) | 38 (6%) | 522 (8%) |
| Left bundle branch block | 45 (15%) | 40 (13%) | 85 (14%) | 865 (13%) |
| Treatment at randomization | ||||
| β-blocker | 281 (92%) | 281 (92%) | 562 (92%) | 5820 (90%) |
| ACE inhibitor | 243 (80%) | 255 (83%) | 498 (82%) | 5116 (79%) |
| ARB | 51 (17%) | 36 (12%) | 87 (14%) | 927 (14%) |
| Diuretic (excluding antialdosterone) | 264 (87%) | 266 (87%) | 530 (87%) | 5414 (83%) |
| Aldosterone antagonist | 224 (74%) | 218 (71%) | 442 (72%) | 3922 (60%) |
| Digitalis | 83 (27%) | 99 (32%) | 182 (30%) | 1416 (22%) |
| Devices | ||||
| At least one device | 10 (3%) | 13 (4%) | 23 (4%) | 244 (4%) |
| ICD | 9 (3%) | 11 (4%) | 20 (3%) | 207 (3%) |
Data are numbers of patients (%) or means ± SD.
BMI, body mass index; bpm, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ICD, implantable cardioverter defibrillator; MDRD, Modification of Diet in Renal Disease Study equation.
Baseline characteristics of the substudy population (included set) vs. the population of the main SHIFT study6 (randomized set)
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| Demographic parameters | ||||
| Age (years) | 60.4 ± 10.9 | 59.1 ± 11.1 | 59.7 ± 11.0 | 60.4 ± 11.4 |
| Sex (male) | 244 (80%) | 252 (82%) | 496 (81%) | 4970 (76%) |
| Caucasian | 276 (91%) | 281 (92%) | 557 (91%) | 5771 (89%) |
| Current smoker | 48 (16%) | 48 (16%) | 96 (16%) | 1118 (17%) |
| BMI (kg/m2) | 27.7 ± 4.5 | 27.7 ± 4.9 | 27.7 ± 4.7 | 28.0 ± 5.1 |
| Cardiac parameters | ||||
| Heart rate (bpm) | 78.4 ± 9.0 | 78.8 ± 9.2 | 78.6 ± 9.1 | 79.9 ± 9.6 |
| Heart rate ≥77 bpm | 137 (45%) | 129 (42%) | 266 (44%) | 3357 (52%) |
| Sitting SBP (mm Hg) | 120.5 ± 15.9 | 119.1 ± 15.4 | 119.8 ± 15.6 | 121.7 ± 16.0 |
| Sitting DBP (mm Hg) | 75.2 ± 9.3 | 75.4 ± 9.0 | 75.3 ± 9.1 | 75.7 ± 9.5 |
| eGFR (MDRD formula) (mL/min/1.73 m2) | 73.7 ± 20.6 | 75.8 ± 25.3 | 74.7 ± 23.1 | 74.7 ± 23.0 |
| NYHA class | ||||
| Class II | 146 (48%) | 140 (46%) | 286 (47%) | 3169 (49%) |
| Class III | 156 (51%) | 164 (53%) | 320 (52%) | 3223 (50%) |
| Class IV | 2 (0.7%) | 3 (1.0%) | 5 (0.8%) | 111 (1.7%) |
| Medical history | ||||
| Duration of heart failure (years) | 3.5 ± 4.0 | 3.5 ± 4.5 | 3.5 ± 4.3 | 3.5 ± 4.2 |
| Primary cause of heart failure | ||||
| Ischaemic | 205 (67%) | 200 (65%) | 405 (66%) | 4418 (68%) |
| Non-ischaemic | 99 (33%) | 107 (35%) | 206 (34%) | 2087 (32%) |
| Myocardial infarction | 180 (59%) | 178 (58%) | 358 (59%) | 3666 (56%) |
| Hypertension | 179 (59%) | 175 (57%) | 354 (58%) | 4314 (66%) |
| Diabetes | 96 (32%) | 98 (32%) | 194 (32%) | 1979 (30%) |
| Previous stroke | 25 (8%) | 28 (9%) | 53 (9%) | 522 (8%) |
| History atrial fibrillation and/or atrial flutter | 18 (6%) | 20 (7%) | 38 (6%) | 522 (8%) |
| Left bundle branch block | 45 (15%) | 40 (13%) | 85 (14%) | 865 (13%) |
| Treatment at randomization | ||||
| β-blocker | 281 (92%) | 281 (92%) | 562 (92%) | 5820 (90%) |
| ACE inhibitor | 243 (80%) | 255 (83%) | 498 (82%) | 5116 (79%) |
| ARB | 51 (17%) | 36 (12%) | 87 (14%) | 927 (14%) |
| Diuretic (excluding antialdosterone) | 264 (87%) | 266 (87%) | 530 (87%) | 5414 (83%) |
| Aldosterone antagonist | 224 (74%) | 218 (71%) | 442 (72%) | 3922 (60%) |
| Digitalis | 83 (27%) | 99 (32%) | 182 (30%) | 1416 (22%) |
| Devices | ||||
| At least one device | 10 (3%) | 13 (4%) | 23 (4%) | 244 (4%) |
| ICD | 9 (3%) | 11 (4%) | 20 (3%) | 207 (3%) |
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| Demographic parameters | ||||
| Age (years) | 60.4 ± 10.9 | 59.1 ± 11.1 | 59.7 ± 11.0 | 60.4 ± 11.4 |
| Sex (male) | 244 (80%) | 252 (82%) | 496 (81%) | 4970 (76%) |
| Caucasian | 276 (91%) | 281 (92%) | 557 (91%) | 5771 (89%) |
| Current smoker | 48 (16%) | 48 (16%) | 96 (16%) | 1118 (17%) |
| BMI (kg/m2) | 27.7 ± 4.5 | 27.7 ± 4.9 | 27.7 ± 4.7 | 28.0 ± 5.1 |
| Cardiac parameters | ||||
| Heart rate (bpm) | 78.4 ± 9.0 | 78.8 ± 9.2 | 78.6 ± 9.1 | 79.9 ± 9.6 |
| Heart rate ≥77 bpm | 137 (45%) | 129 (42%) | 266 (44%) | 3357 (52%) |
| Sitting SBP (mm Hg) | 120.5 ± 15.9 | 119.1 ± 15.4 | 119.8 ± 15.6 | 121.7 ± 16.0 |
| Sitting DBP (mm Hg) | 75.2 ± 9.3 | 75.4 ± 9.0 | 75.3 ± 9.1 | 75.7 ± 9.5 |
| eGFR (MDRD formula) (mL/min/1.73 m2) | 73.7 ± 20.6 | 75.8 ± 25.3 | 74.7 ± 23.1 | 74.7 ± 23.0 |
| NYHA class | ||||
| Class II | 146 (48%) | 140 (46%) | 286 (47%) | 3169 (49%) |
| Class III | 156 (51%) | 164 (53%) | 320 (52%) | 3223 (50%) |
| Class IV | 2 (0.7%) | 3 (1.0%) | 5 (0.8%) | 111 (1.7%) |
| Medical history | ||||
| Duration of heart failure (years) | 3.5 ± 4.0 | 3.5 ± 4.5 | 3.5 ± 4.3 | 3.5 ± 4.2 |
| Primary cause of heart failure | ||||
| Ischaemic | 205 (67%) | 200 (65%) | 405 (66%) | 4418 (68%) |
| Non-ischaemic | 99 (33%) | 107 (35%) | 206 (34%) | 2087 (32%) |
| Myocardial infarction | 180 (59%) | 178 (58%) | 358 (59%) | 3666 (56%) |
| Hypertension | 179 (59%) | 175 (57%) | 354 (58%) | 4314 (66%) |
| Diabetes | 96 (32%) | 98 (32%) | 194 (32%) | 1979 (30%) |
| Previous stroke | 25 (8%) | 28 (9%) | 53 (9%) | 522 (8%) |
| History atrial fibrillation and/or atrial flutter | 18 (6%) | 20 (7%) | 38 (6%) | 522 (8%) |
| Left bundle branch block | 45 (15%) | 40 (13%) | 85 (14%) | 865 (13%) |
| Treatment at randomization | ||||
| β-blocker | 281 (92%) | 281 (92%) | 562 (92%) | 5820 (90%) |
| ACE inhibitor | 243 (80%) | 255 (83%) | 498 (82%) | 5116 (79%) |
| ARB | 51 (17%) | 36 (12%) | 87 (14%) | 927 (14%) |
| Diuretic (excluding antialdosterone) | 264 (87%) | 266 (87%) | 530 (87%) | 5414 (83%) |
| Aldosterone antagonist | 224 (74%) | 218 (71%) | 442 (72%) | 3922 (60%) |
| Digitalis | 83 (27%) | 99 (32%) | 182 (30%) | 1416 (22%) |
| Devices | ||||
| At least one device | 10 (3%) | 13 (4%) | 23 (4%) | 244 (4%) |
| ICD | 9 (3%) | 11 (4%) | 20 (3%) | 207 (3%) |
Data are numbers of patients (%) or means ± SD.
BMI, body mass index; bpm, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ICD, implantable cardioverter defibrillator; MDRD, Modification of Diet in Renal Disease Study equation.
Types and doses of beta-blockers at baseline in the substudy population (included set) vs. the population of the main SHIFT study6 (randomized set)
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| β-blockers | 281 (92%) | 281 (91.5%) | 562 (92%) | 5820 (90%) |
| Carvedilol | 160 (57%) | 149 (53%) | 309 (55%) | 2604 (45%) |
| Bisoprolol | 46 (16%) | 60 (21%) | 106 (19%) | 1486 (26%) |
| Metoprolol succinate | 48 (17%) | 45 (16%) | 93 (17%) | 815 (14%) |
| Metoprolol tartrate | 16 (6%) | 19 (7%) | 35 (6%) | 618 (11%) |
| Nebivolol | 9 (3%) | 9 (3%) | 18 (3%) | 198 (3%) |
| Other | 2 (0.7%) | – | 2 (0.4%) | 107 (1.8%) |
| Mean daily dosage of β-blocker (mg) | ||||
| Carvedilol | 23.5 ± 16.0 | 23.5 ± 17.1 | 23.5 ± 16.5 | 25.0 ± 17.8 |
| Bisoprolol | 6.2 ± 3.2 | 5.7 ± 3.2 | 5.9 ± 3.2 | 6.2 ± 3.3 |
| Metoprolol succinate | 131.3 ± 80.2 | 131.9 ± 73.3 | 131.6 ± 76.5 | 89.9 ± 60.0 |
| Metoprolol tartrate | 72.7 ± 46.2 | 115.8 ± 50.8 | 96.1 ± 52.8 | 69.1 ± 47.4 |
| Nebivolol | 6.4 ± 2.8 | 4.3 ± 2.6 | 5.4 ± 2.8 | 5.9 ± 2.9 |
| Patients at target dose of β-blocker | 76 (27%) | 87 (31%) | 163 (29%) | 1488 (26%) |
| Patients at ≥50% target dose of β-blocker | 159 (57%) | 154 (55%) | 313 (56%) | 3181 (56%) |
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| β-blockers | 281 (92%) | 281 (91.5%) | 562 (92%) | 5820 (90%) |
| Carvedilol | 160 (57%) | 149 (53%) | 309 (55%) | 2604 (45%) |
| Bisoprolol | 46 (16%) | 60 (21%) | 106 (19%) | 1486 (26%) |
| Metoprolol succinate | 48 (17%) | 45 (16%) | 93 (17%) | 815 (14%) |
| Metoprolol tartrate | 16 (6%) | 19 (7%) | 35 (6%) | 618 (11%) |
| Nebivolol | 9 (3%) | 9 (3%) | 18 (3%) | 198 (3%) |
| Other | 2 (0.7%) | – | 2 (0.4%) | 107 (1.8%) |
| Mean daily dosage of β-blocker (mg) | ||||
| Carvedilol | 23.5 ± 16.0 | 23.5 ± 17.1 | 23.5 ± 16.5 | 25.0 ± 17.8 |
| Bisoprolol | 6.2 ± 3.2 | 5.7 ± 3.2 | 5.9 ± 3.2 | 6.2 ± 3.3 |
| Metoprolol succinate | 131.3 ± 80.2 | 131.9 ± 73.3 | 131.6 ± 76.5 | 89.9 ± 60.0 |
| Metoprolol tartrate | 72.7 ± 46.2 | 115.8 ± 50.8 | 96.1 ± 52.8 | 69.1 ± 47.4 |
| Nebivolol | 6.4 ± 2.8 | 4.3 ± 2.6 | 5.4 ± 2.8 | 5.9 ± 2.9 |
| Patients at target dose of β-blocker | 76 (27%) | 87 (31%) | 163 (29%) | 1488 (26%) |
| Patients at ≥50% target dose of β-blocker | 159 (57%) | 154 (55%) | 313 (56%) | 3181 (56%) |
Data are numbers of patients (%) or means ± SD.
Types and doses of beta-blockers at baseline in the substudy population (included set) vs. the population of the main SHIFT study6 (randomized set)
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| β-blockers | 281 (92%) | 281 (91.5%) | 562 (92%) | 5820 (90%) |
| Carvedilol | 160 (57%) | 149 (53%) | 309 (55%) | 2604 (45%) |
| Bisoprolol | 46 (16%) | 60 (21%) | 106 (19%) | 1486 (26%) |
| Metoprolol succinate | 48 (17%) | 45 (16%) | 93 (17%) | 815 (14%) |
| Metoprolol tartrate | 16 (6%) | 19 (7%) | 35 (6%) | 618 (11%) |
| Nebivolol | 9 (3%) | 9 (3%) | 18 (3%) | 198 (3%) |
| Other | 2 (0.7%) | – | 2 (0.4%) | 107 (1.8%) |
| Mean daily dosage of β-blocker (mg) | ||||
| Carvedilol | 23.5 ± 16.0 | 23.5 ± 17.1 | 23.5 ± 16.5 | 25.0 ± 17.8 |
| Bisoprolol | 6.2 ± 3.2 | 5.7 ± 3.2 | 5.9 ± 3.2 | 6.2 ± 3.3 |
| Metoprolol succinate | 131.3 ± 80.2 | 131.9 ± 73.3 | 131.6 ± 76.5 | 89.9 ± 60.0 |
| Metoprolol tartrate | 72.7 ± 46.2 | 115.8 ± 50.8 | 96.1 ± 52.8 | 69.1 ± 47.4 |
| Nebivolol | 6.4 ± 2.8 | 4.3 ± 2.6 | 5.4 ± 2.8 | 5.9 ± 2.9 |
| Patients at target dose of β-blocker | 76 (27%) | 87 (31%) | 163 (29%) | 1488 (26%) |
| Patients at ≥50% target dose of β-blocker | 159 (57%) | 154 (55%) | 313 (56%) | 3181 (56%) |
| . | SHIFT echocardiography substudy . | Main study (n = 6505) . | ||
|---|---|---|---|---|
| . | Ivabradine (n = 304) . | Placebo (n = 307) . | All (n = 611) . | . |
| β-blockers | 281 (92%) | 281 (91.5%) | 562 (92%) | 5820 (90%) |
| Carvedilol | 160 (57%) | 149 (53%) | 309 (55%) | 2604 (45%) |
| Bisoprolol | 46 (16%) | 60 (21%) | 106 (19%) | 1486 (26%) |
| Metoprolol succinate | 48 (17%) | 45 (16%) | 93 (17%) | 815 (14%) |
| Metoprolol tartrate | 16 (6%) | 19 (7%) | 35 (6%) | 618 (11%) |
| Nebivolol | 9 (3%) | 9 (3%) | 18 (3%) | 198 (3%) |
| Other | 2 (0.7%) | – | 2 (0.4%) | 107 (1.8%) |
| Mean daily dosage of β-blocker (mg) | ||||
| Carvedilol | 23.5 ± 16.0 | 23.5 ± 17.1 | 23.5 ± 16.5 | 25.0 ± 17.8 |
| Bisoprolol | 6.2 ± 3.2 | 5.7 ± 3.2 | 5.9 ± 3.2 | 6.2 ± 3.3 |
| Metoprolol succinate | 131.3 ± 80.2 | 131.9 ± 73.3 | 131.6 ± 76.5 | 89.9 ± 60.0 |
| Metoprolol tartrate | 72.7 ± 46.2 | 115.8 ± 50.8 | 96.1 ± 52.8 | 69.1 ± 47.4 |
| Nebivolol | 6.4 ± 2.8 | 4.3 ± 2.6 | 5.4 ± 2.8 | 5.9 ± 2.9 |
| Patients at target dose of β-blocker | 76 (27%) | 87 (31%) | 163 (29%) | 1488 (26%) |
| Patients at ≥50% target dose of β-blocker | 159 (57%) | 154 (55%) | 313 (56%) | 3181 (56%) |
Data are numbers of patients (%) or means ± SD.
Median treatment duration during the substudy was 8.1 months (IQR, 7.8–8.3) and median follow-up after the 8-month echocardiogram was 16.1 months (IQR, 13–19.9). Mean dosage of ivabradine during the substudy was 6.0 ± 1.6 mg bid. Heart rate was reduced by 14.7 ± 11.4 bpm in the ivabradine group to 63.5 ± 9.5 bpm at 8 months vs. by 5.8 ± 10.8 bpm to 72.2 ± 12.4 bpm with placebo.
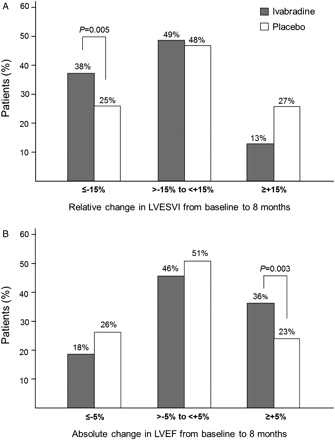
Mean baseline LVESVI was similar in the ivabradine and placebo groups (65.2 ± 29.1 and 63.6 ± 30.1 mL/m2, respectively). Treatment with ivabradine for 8 months was associated with a significant reduction in LVESVI vs. placebo [−7.0 ± 16.3 vs. −0.9 ± 17.1 mL/m2, estimate (SE) −5.8 (1.6), 95% CI −8.8 to −2.7, P< 0.001, Table 3]. More patients in the ivabradine group had a 15% or more decrease in LVESVI than in the placebo group (38 vs. 25%, respectively, P= 0.005) (Figure 2). The ivabradine-associated improvement in LVESVI was consistent in all the prespecified subgroups: ischaemic vs. non-ischaemic HF (estimate of treatment-placebo difference −5.51 vs. −7.61), with or without half target beta-blocker dose at randomization (−4.39 vs. −7.89), and baseline LVEF above and below 32% (−6.08 vs. −5.77).
Echocardiographic parameters at baseline, 8 months, and their changes in the substudy
| Variable . | Ivabradine . | Placebo . | Treatment effect . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Baseline . | 8 months . | Change . | n . | Baseline . | 8 months . | Change . | E (SE), 95% CI . | P-value . |
| Primary endpoint | ||||||||||
| LVESVI (mL/m2) | 208 | 65.2 ± 29.1 | 58.2 ± 28.3 | −7.0 ± 16.3 | 203 | 63.6 ± 30.1 | 62.8 ± 28.7 | −0.9 ± 17.1 | −5.8 (1.6), −8.8 to −2.7 | <0.001 |
| Secondary endpoints | ||||||||||
| LVESV (mL) | 208 | 123.8 ± 55.6 | 110.8 ± 54.6 | −13.0 ± 31.6 | 203 | 122.2 ± 59.8 | 120.9 ± 56.4 | −1.3 ± 32.8 | −11.2 (3.0), −17.1 to −5.4 | <0.001 |
| LVEDVI (mL/m2) | 204 | 93.9 ± 32.8 | 85.9 ± 30.9 | −7.9 ± 18.9 | 199 | 90.8 ± 33.1 | 89.0 ± 31.6 | −1.8 ± 19.0 | −5.5 (1.8), −8.9 to −2.0 | 0.002 |
| LVEDV (mL) | 204 | 178.4 ± 63.4 | 163.7 ± 60.6 | −14.7± 36.4 | 199 | 174.7 ± 67.6 | 171.7 ± 63.8 | −2.9 ± 36.8 | −10.9 (3.4), −17.6 to −4.2 | 0.001 |
| LVEF (%) | 204 | 32.3 ± 9.1 | 34.7 ± 10.2 | 2.4 ± 7.7 | 199 | 31.6 ± 9.3 | 31.5 ± 10.0 | −0.1 ± 8.0 | 2.7 (0.8), 1.3 to 4.2 | <0.001 |
| Variable . | Ivabradine . | Placebo . | Treatment effect . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Baseline . | 8 months . | Change . | n . | Baseline . | 8 months . | Change . | E (SE), 95% CI . | P-value . |
| Primary endpoint | ||||||||||
| LVESVI (mL/m2) | 208 | 65.2 ± 29.1 | 58.2 ± 28.3 | −7.0 ± 16.3 | 203 | 63.6 ± 30.1 | 62.8 ± 28.7 | −0.9 ± 17.1 | −5.8 (1.6), −8.8 to −2.7 | <0.001 |
| Secondary endpoints | ||||||||||
| LVESV (mL) | 208 | 123.8 ± 55.6 | 110.8 ± 54.6 | −13.0 ± 31.6 | 203 | 122.2 ± 59.8 | 120.9 ± 56.4 | −1.3 ± 32.8 | −11.2 (3.0), −17.1 to −5.4 | <0.001 |
| LVEDVI (mL/m2) | 204 | 93.9 ± 32.8 | 85.9 ± 30.9 | −7.9 ± 18.9 | 199 | 90.8 ± 33.1 | 89.0 ± 31.6 | −1.8 ± 19.0 | −5.5 (1.8), −8.9 to −2.0 | 0.002 |
| LVEDV (mL) | 204 | 178.4 ± 63.4 | 163.7 ± 60.6 | −14.7± 36.4 | 199 | 174.7 ± 67.6 | 171.7 ± 63.8 | −2.9 ± 36.8 | −10.9 (3.4), −17.6 to −4.2 | 0.001 |
| LVEF (%) | 204 | 32.3 ± 9.1 | 34.7 ± 10.2 | 2.4 ± 7.7 | 199 | 31.6 ± 9.3 | 31.5 ± 10.0 | −0.1 ± 8.0 | 2.7 (0.8), 1.3 to 4.2 | <0.001 |
Data are numbers of patients (%) or means ± SD. Parametric approach with adjustment for country, beta-blocker intake at randomization, and baseline value. E (SE), estimate (standard error of treatment effect); CI, confidence interval.
LVESV, left ventricular end-systolic volume; LVESVI, left ventricular end-systolic volume index; LVEDV, left ventricular end-diastolic volume; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction.
Echocardiographic parameters at baseline, 8 months, and their changes in the substudy
| Variable . | Ivabradine . | Placebo . | Treatment effect . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Baseline . | 8 months . | Change . | n . | Baseline . | 8 months . | Change . | E (SE), 95% CI . | P-value . |
| Primary endpoint | ||||||||||
| LVESVI (mL/m2) | 208 | 65.2 ± 29.1 | 58.2 ± 28.3 | −7.0 ± 16.3 | 203 | 63.6 ± 30.1 | 62.8 ± 28.7 | −0.9 ± 17.1 | −5.8 (1.6), −8.8 to −2.7 | <0.001 |
| Secondary endpoints | ||||||||||
| LVESV (mL) | 208 | 123.8 ± 55.6 | 110.8 ± 54.6 | −13.0 ± 31.6 | 203 | 122.2 ± 59.8 | 120.9 ± 56.4 | −1.3 ± 32.8 | −11.2 (3.0), −17.1 to −5.4 | <0.001 |
| LVEDVI (mL/m2) | 204 | 93.9 ± 32.8 | 85.9 ± 30.9 | −7.9 ± 18.9 | 199 | 90.8 ± 33.1 | 89.0 ± 31.6 | −1.8 ± 19.0 | −5.5 (1.8), −8.9 to −2.0 | 0.002 |
| LVEDV (mL) | 204 | 178.4 ± 63.4 | 163.7 ± 60.6 | −14.7± 36.4 | 199 | 174.7 ± 67.6 | 171.7 ± 63.8 | −2.9 ± 36.8 | −10.9 (3.4), −17.6 to −4.2 | 0.001 |
| LVEF (%) | 204 | 32.3 ± 9.1 | 34.7 ± 10.2 | 2.4 ± 7.7 | 199 | 31.6 ± 9.3 | 31.5 ± 10.0 | −0.1 ± 8.0 | 2.7 (0.8), 1.3 to 4.2 | <0.001 |
| Variable . | Ivabradine . | Placebo . | Treatment effect . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Baseline . | 8 months . | Change . | n . | Baseline . | 8 months . | Change . | E (SE), 95% CI . | P-value . |
| Primary endpoint | ||||||||||
| LVESVI (mL/m2) | 208 | 65.2 ± 29.1 | 58.2 ± 28.3 | −7.0 ± 16.3 | 203 | 63.6 ± 30.1 | 62.8 ± 28.7 | −0.9 ± 17.1 | −5.8 (1.6), −8.8 to −2.7 | <0.001 |
| Secondary endpoints | ||||||||||
| LVESV (mL) | 208 | 123.8 ± 55.6 | 110.8 ± 54.6 | −13.0 ± 31.6 | 203 | 122.2 ± 59.8 | 120.9 ± 56.4 | −1.3 ± 32.8 | −11.2 (3.0), −17.1 to −5.4 | <0.001 |
| LVEDVI (mL/m2) | 204 | 93.9 ± 32.8 | 85.9 ± 30.9 | −7.9 ± 18.9 | 199 | 90.8 ± 33.1 | 89.0 ± 31.6 | −1.8 ± 19.0 | −5.5 (1.8), −8.9 to −2.0 | 0.002 |
| LVEDV (mL) | 204 | 178.4 ± 63.4 | 163.7 ± 60.6 | −14.7± 36.4 | 199 | 174.7 ± 67.6 | 171.7 ± 63.8 | −2.9 ± 36.8 | −10.9 (3.4), −17.6 to −4.2 | 0.001 |
| LVEF (%) | 204 | 32.3 ± 9.1 | 34.7 ± 10.2 | 2.4 ± 7.7 | 199 | 31.6 ± 9.3 | 31.5 ± 10.0 | −0.1 ± 8.0 | 2.7 (0.8), 1.3 to 4.2 | <0.001 |
Data are numbers of patients (%) or means ± SD. Parametric approach with adjustment for country, beta-blocker intake at randomization, and baseline value. E (SE), estimate (standard error of treatment effect); CI, confidence interval.
LVESV, left ventricular end-systolic volume; LVESVI, left ventricular end-systolic volume index; LVEDV, left ventricular end-diastolic volume; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction.
(A) Relative change in left ventricular end-systolic volume index (LVESVI) and (B) absolute change in left ventricular ejection fraction (LVEF) from baseline to 8 months. The grey and white bars represent percentages of patients reaching echocardiographic criteria for the ivabradine and placebo groups, respectively.
The mean values of the secondary efficacy criteria were similar at baseline in the ivabradine and placebo groups (Table 3). Significant treatment effects were found at 8 months for ivabradine vs. placebo on LVEDVI (P= 0.002), LVESV (P< 0.001), LVEDV (P= 0.001), and LVEF (P< 0.001) (Table 3). Left ventricular ejection fraction was increased by 2.4 ± 7.7% in the ivabradine group but was unchanged in the placebo group (−0.1 ± 8.0%). More than a third (36%) of the patients in the ivabradine group had ≥5% increase in LVEF vs. less than a quarter (23%) of the placebo group (P= 0.003) (Figure 2). Tertiary endpoints were measured in limited numbers of patients. There were no significant changes in left-atrial end-systolic volume index or RV myocardial performance index in either group, while RV s wave peak velocity increased over 8 months with ivabradine and decreased with placebo (data not shown). There were 351 patients who had an evaluation of mitral regurgitation at baseline and 8 months. Of these, mitral regurgitation improved by at least one grade in 18 patients (18/176, 10%) in the ivabradine group vs. 14 patients (14/175, 8%) in the placebo group (P= 0.47). More patients improved by at least one grade of LV diastolic function from baseline to 8 months with ivabradine [26 patients out of 120 fully documented patients (22%)] than with placebo [11 patients out of 106 (10%), P= 0.02].
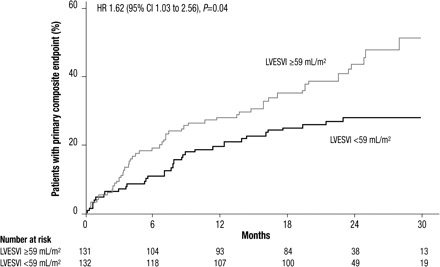
To explore the impact of baseline values of echocardiographic parameters reflecting LV systolic function, we divided the placebo group by median baseline LVESVI (59 mL/m2) and found that the incidence of the primary composite endpoint of hospitalization for worsening HF or cardiovascular mortality was significantly greater in the patients with higher values (HR 1.62, 95% CI 1.03–2.56, P= 0.04, Figure 3). The increase in risk appeared to be driven by both hospitalizations for worsening HF (HR 1.80, 95% CI 1.06–3.07, P= 0.03) and cardiovascular deaths (HR 1.56, 95% CI 0.78–3.10, P= 0.21, Table 4). Similar changes in risk were observed when the placebo group was split by median baseline LVEDVI (85 mL/m2), or for median baseline LVEF (31%), though the differences were not statistically significant for LVEF (Table 4).
Relationship between echocardiography parameters at baseline and the SHIFT primary composite endpoint and its components in the placebo group of the substudy (included set echo)
| . | Number of events . | HR (95% CI) . | P-value . |
|---|---|---|---|
| LVESVI (n = 263) | |||
| SHIFT primary composite endpoint | |||
| ≥59 mL/m2 | 56 | 1.62 (1.03–2.56) | 0.04 |
| <59 mL/m2 | 35 | ||
| Hospitalization for worsening heart failure | |||
| ≥59 mL/m2 | 44 | 1.80 (1.06–3.07) | 0.03 |
| <59 mL/m2 | 25 | ||
| Cardiovascular deatha | |||
| ≥59 mL/m2 | 28 | 1.56 (0.78–3.10) | 0.21 |
| <59 mL/m2 | 15 | ||
| LVEDVI (n = 257) | |||
| SHIFT primary composite endpoint | |||
| ≥85 mL/m2 | 56 | 1.75 (1.11–2.75) | 0.02 |
| <85 mL/m2 | 34 | ||
| Hospitalization for worsening heart failure | |||
| ≥85 mL/m2 | 44 | 1.96 (1.15–3.34) | 0.01 |
| <85 mL/m2 | 24 | ||
| Cardiovascular death | |||
| ≥85 mL/m2 | 28 | 1.65 (0.84–3.23) | 0.14 |
| <85 mL/m2 | 15 | ||
| LVEF (n = 257) | |||
| SHIFT primary composite endpoint | |||
| <31% | 50 | 1.21 (0.78–1.88) | 0.40 |
| ≥31% | 40 | ||
| Hospitalization for worsening heart failure | |||
| <31% | 39 | 1.28 (0.77–2.13) | 0.34 |
| ≥31% | 29 | ||
| Cardiovascular death | |||
| <31% | 26 | 1.29 (0.67–2.51) | 0.45 |
| ≥31% | 17 | ||
| . | Number of events . | HR (95% CI) . | P-value . |
|---|---|---|---|
| LVESVI (n = 263) | |||
| SHIFT primary composite endpoint | |||
| ≥59 mL/m2 | 56 | 1.62 (1.03–2.56) | 0.04 |
| <59 mL/m2 | 35 | ||
| Hospitalization for worsening heart failure | |||
| ≥59 mL/m2 | 44 | 1.80 (1.06–3.07) | 0.03 |
| <59 mL/m2 | 25 | ||
| Cardiovascular deatha | |||
| ≥59 mL/m2 | 28 | 1.56 (0.78–3.10) | 0.21 |
| <59 mL/m2 | 15 | ||
| LVEDVI (n = 257) | |||
| SHIFT primary composite endpoint | |||
| ≥85 mL/m2 | 56 | 1.75 (1.11–2.75) | 0.02 |
| <85 mL/m2 | 34 | ||
| Hospitalization for worsening heart failure | |||
| ≥85 mL/m2 | 44 | 1.96 (1.15–3.34) | 0.01 |
| <85 mL/m2 | 24 | ||
| Cardiovascular death | |||
| ≥85 mL/m2 | 28 | 1.65 (0.84–3.23) | 0.14 |
| <85 mL/m2 | 15 | ||
| LVEF (n = 257) | |||
| SHIFT primary composite endpoint | |||
| <31% | 50 | 1.21 (0.78–1.88) | 0.40 |
| ≥31% | 40 | ||
| Hospitalization for worsening heart failure | |||
| <31% | 39 | 1.28 (0.77–2.13) | 0.34 |
| ≥31% | 29 | ||
| Cardiovascular death | |||
| <31% | 26 | 1.29 (0.67–2.51) | 0.45 |
| ≥31% | 17 | ||
LVESVI, left ventricular end-systolic volume index; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; HR, hazard ratio; CI, confidence interval.
Relationship between echocardiography parameters at baseline and the SHIFT primary composite endpoint and its components in the placebo group of the substudy (included set echo)
| . | Number of events . | HR (95% CI) . | P-value . |
|---|---|---|---|
| LVESVI (n = 263) | |||
| SHIFT primary composite endpoint | |||
| ≥59 mL/m2 | 56 | 1.62 (1.03–2.56) | 0.04 |
| <59 mL/m2 | 35 | ||
| Hospitalization for worsening heart failure | |||
| ≥59 mL/m2 | 44 | 1.80 (1.06–3.07) | 0.03 |
| <59 mL/m2 | 25 | ||
| Cardiovascular deatha | |||
| ≥59 mL/m2 | 28 | 1.56 (0.78–3.10) | 0.21 |
| <59 mL/m2 | 15 | ||
| LVEDVI (n = 257) | |||
| SHIFT primary composite endpoint | |||
| ≥85 mL/m2 | 56 | 1.75 (1.11–2.75) | 0.02 |
| <85 mL/m2 | 34 | ||
| Hospitalization for worsening heart failure | |||
| ≥85 mL/m2 | 44 | 1.96 (1.15–3.34) | 0.01 |
| <85 mL/m2 | 24 | ||
| Cardiovascular death | |||
| ≥85 mL/m2 | 28 | 1.65 (0.84–3.23) | 0.14 |
| <85 mL/m2 | 15 | ||
| LVEF (n = 257) | |||
| SHIFT primary composite endpoint | |||
| <31% | 50 | 1.21 (0.78–1.88) | 0.40 |
| ≥31% | 40 | ||
| Hospitalization for worsening heart failure | |||
| <31% | 39 | 1.28 (0.77–2.13) | 0.34 |
| ≥31% | 29 | ||
| Cardiovascular death | |||
| <31% | 26 | 1.29 (0.67–2.51) | 0.45 |
| ≥31% | 17 | ||
| . | Number of events . | HR (95% CI) . | P-value . |
|---|---|---|---|
| LVESVI (n = 263) | |||
| SHIFT primary composite endpoint | |||
| ≥59 mL/m2 | 56 | 1.62 (1.03–2.56) | 0.04 |
| <59 mL/m2 | 35 | ||
| Hospitalization for worsening heart failure | |||
| ≥59 mL/m2 | 44 | 1.80 (1.06–3.07) | 0.03 |
| <59 mL/m2 | 25 | ||
| Cardiovascular deatha | |||
| ≥59 mL/m2 | 28 | 1.56 (0.78–3.10) | 0.21 |
| <59 mL/m2 | 15 | ||
| LVEDVI (n = 257) | |||
| SHIFT primary composite endpoint | |||
| ≥85 mL/m2 | 56 | 1.75 (1.11–2.75) | 0.02 |
| <85 mL/m2 | 34 | ||
| Hospitalization for worsening heart failure | |||
| ≥85 mL/m2 | 44 | 1.96 (1.15–3.34) | 0.01 |
| <85 mL/m2 | 24 | ||
| Cardiovascular death | |||
| ≥85 mL/m2 | 28 | 1.65 (0.84–3.23) | 0.14 |
| <85 mL/m2 | 15 | ||
| LVEF (n = 257) | |||
| SHIFT primary composite endpoint | |||
| <31% | 50 | 1.21 (0.78–1.88) | 0.40 |
| ≥31% | 40 | ||
| Hospitalization for worsening heart failure | |||
| <31% | 39 | 1.28 (0.77–2.13) | 0.34 |
| ≥31% | 29 | ||
| Cardiovascular death | |||
| <31% | 26 | 1.29 (0.67–2.51) | 0.45 |
| ≥31% | 17 | ||
LVESVI, left ventricular end-systolic volume index; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; HR, hazard ratio; CI, confidence interval.
Kaplan–Meier cumulative event curves for the SHIFT primary composite endpoint of cardiovascular death or hospitalization for worsening heart failure in the placebo group split by median left ventricular end-systolic volume index (LVESVI) ≥59 vs. <59 mL/m2.
In the SHIFT echocardiography substudy population, there were 102 primary composite endpoint events (81 hospitalizations for worsening HF and 21 cardiovascular deaths), 63 of which occurred after 8 months. This relatively low number of events provided little power to detect an effect of treatment on outcomes according to echocardiographic parameters. To obtain some measure of the effect, we divided the ivabradine group and the placebo group by tertiles of change in LVESVI and determined the incidence of primary composite endpoint after 8 months for each third. Despite few events in this exploratory analysis, patients in the lowest third, i.e. those with the greatest relative reductions in LVESVI, had numerically lower event rates than those with smaller relative reductions or relative increases (data not shown).
The relationships between changes in heart rate and both changes in LV volumes and LVEF were explored. There was an inverse relation between the change in heart rate and the change in LVEF (r = −0.17, P= 0.0006); expressed differently, larger decreases in heart rate were associated with greater increases in LVEF. In contrast, there was no significant relationship between changes in heart rate and changes in LV volumes for all patients. However, when split by baseline median heart rate, those patients who were above the median of 77 bpm had larger LV volumes (LVESVI: 68.1 ± 29.3 vs. 61.8 ± 30.3 mL/m2, P= 0.019) and lower LVEF (29.4 ± 9.0 vs. 33.1 ± 8.8%, P< 0.001) at baseline.
Discussion
Our results indicate a reversal of cardiac remodelling with ivabradine, with significant reductions in LV volumes and an increase in LVEF over 8 months of treatment. These changes were consistent in the prespecified subgroups, irrespective of baseline LVEF, beta-blocker intake, and aetiology of HF. Moreover, these results occurred despite treatment with beta-blockers and RAAS antagonists, each used in more than 90% of patients.
Our results have important clinical implications since cardiac remodelling is a central feature of the progression of HF. We explored the association between the risk of clinical cardiovascular adverse outcomes and echocardiographic parameters in a series of complementary analyses. Left ventricular end-systolic volume index ≥59 mL/m2 at baseline was associated with a 62% increase in relative risk for the SHIFT primary composite endpoint of cardiovascular death or hospitalization for worsening HF vs. patients with LVESVI <59 mL/m2. Using the median to divide these data may have diluted the relation to the SHIFT primary composite endpoint observed at higher LV volumes. In addition, the patients with the largest treatment-related reductions in LVESVI at follow-up had a numerically lower rate of the SHIFT primary composite endpoint than patients with smaller reductions in LVESVI. This exploratory analysis suggests that there is a link between treatment-related improvement in LV function and prognosis in HF patients, and is consistent with the observation that short-term effects of drugs or devices are associated with long-term effects on mortality.5
The study provides further support for the role of heart rate reduction with ivabradine to improve cardiac remodelling. Compared with those with lower heart rates, patients with a heart rate ≥77 bpm at baseline had larger LVESVI and lower LVEF at baseline. Furthermore, there was a statistically significant albeit weak inverse relationship between the change in heart rate and the change in LVEF, which means that larger decreases in heart rate during follow-up were associated with greater increases in LVEF. A positive effect of ivabradine on LV remodelling was also demonstrated in the echocardiographic substudy of the BEAUTIFUL (morBidity-mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction) trial, in a different population of patients with stable coronary artery disease and LV systolic dysfunction.9 Results in experimental studies are consistent with the effects of ivabradine on cardiac remodelling that we observed. In a rat model of chronic mild HF, ivabradine preserved cardiac output and improved LV function and geometry.10 These changes were linked to modifications in the extracellular matrix and in cardiac myocyte function. Ivabradine also has had beneficial effects on the global cardiac remodelling process involved in HF, including optimization of energy consumption, reverse electrophysiological and structural cardiac remodelling.11 Similar effects with ivabradine have been found by other workers in a rat model of chronic severe HF, including reductions in fibrosis, local RAAS stimulation, and sympathetic drive, as well as improvement in endothelial function.12–14 Experiments in a canine model also indicated a positive impact on sarcoplasmic reticulum calcium overload.15 Together, these experimental results support a beneficial role of ivabradine on LV remodelling and function that may contribute to the reduction in cardiac morbidity and mortality found in patients with HF.
Limitations
The reduction in morbidity and mortality with RAAS antagonists and beta-blockade in HF is associated with improvement in LV function.16–20 Our analysis was not designed to clarify the time-course of treatment effects and could not evaluate the acute effect of ivabradine. In the absence of acute measurements, the degree to which volumetric changes are influenced by heart rate itself cannot be determined. Our finding of improved LV function with ivabradine shows that further improvement in reverse cardiac remodelling and slowing of disease progression can be achieved, in addition to that obtained with background therapy for HF. Patients needed to be treated with a beta-blocker to be eligible for this study, and the SHIFT trial sought to exclude patients who were not receiving guidelines-driven beta-blocker dosing. Given the beta-blocker dosage observed in SHIFT (which was similar to other recently published data21) and the effect on LV ejection fraction of higher doses,22 further investigation would be required to shed light on the comparison between ivabradine and more aggressive beta-blockade (when tolerated). Our data were recorded in patients with heart rates ≥70 bpm and in sinus rhythm and predominantly in men, which may limit the generalization of our results to all patients with HF. Another limitation of our study is that about a third of patients were excluded from the analysis, usually for reasons related to the quality or collection of the echocardiographic recordings.
Conclusions
Heart rate reduction with ivabradine reverses LV remodelling in patients with HF and LV systolic dysfunction. Treatment with ivabradine is associated with marked reductions in LV volumes and a significant improvement in LVEF, therefore suggesting that it is modifying disease progression in patients with HF.
Supplementary material
Funding
This work was supported by Servier, France. Funding to pay the Open Access publication charges for this article was provided by Servier.
Conflict of interest: All authors have received fees, research grants, or both from Servier.