-
PDF
- Split View
-
Views
-
Cite
Cite
Pablo Rengifo-Moreno, Igor F. Palacios, Parichart Junpaparp, Christian F. Witzke, D. Lynn Morris, Abel Romero-Corral, Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials, European Heart Journal, Volume 34, Issue 43, 14 November 2013, Pages 3342–3352, https://doi.org/10.1093/eurheartj/eht285
Close - Share Icon Share
Abstract
In patients with cryptogenic stroke, transcatheter (TC) closure of a patent foramen ovale (PFO) has not been shown to better prevent recurrent vascular events than medical therapy. However, randomized controlled trials (RCT) to date have included few vascular events, and lack of power has been raised as an important concern.
To conduct a systematic review and meta-analysis of existing RCT published studies assessing the recurrence of vascular events after TC PFO closure when compared to medical therapy.
Using the search terms “patent foramen ovale”, “PFO”, “stroke”, “percutaneous closure” and “transcatheter closure”, Medline, Pubmed, Embase, and Cochrane databases were reviewed from inception through April 2013, with no language restrictions. Only studies in adult humans were considered. Additional references were obtained from the bibliographies of studies reviewed. The following criteria were used for study selection: 1) randomized controlled trial, 2) subjects were adult patients with cryptogenic stroke who were randomized to TC PFO closure or medical treatment (antiplatelet therapy and/or anticoagulation), and 3) reported outcomes included cardiac death, all death, stroke, transient ischemic attack, and peripheral embolism. Methodological and descriptive data, adverse events (including raw data and risk estimates), as well as procedural success and complications were abstracted in duplicate from each study independently, and agreement was tested. We followed rigorously the recommended guidelines for reporting and conducting and assessing quality of meta-analysis of RCT. The primary endpoints pre-specified in advance were recurrent vascular events, and composite endpoint of death, and recurrent vascular events.
Three studies were identified as meeting selection criteria. These included a total of 2,303 patients, with 1,150 patients randomized to TC PFO closure and 1,153 patients randomized to medical therapy. Mean follow-up was 3.5 years. Baseline characteristics (age, sex, and cardiovascular risk factors) were similar across studies. Intention-to-treat analyses showed a statistically significant risk reduction in stroke and/or transient ischemic attack in the TC PFO closure group when compared to medical treatment, pooled HR = 0.59, 95%CI (0.36-0.97), P = 0.04. The combined outcome of death, and vascular events, showed a borderline statistically significant benefit for TC PFO closure when compared to medical treatment, pooled HR = 0.67, 95%CI (0.44–1.00), P = 0.05 Subjects with a substantial PFO shunt seem to benefit the most with TC PFO closure, pooled HR = 0.35, 95%CI (0.12–1.03), P = 0.06, however, it did not reach statistical significance.
These results suggest that in patients with cryptogenic stroke, TC PFO closure may be beneficial in reducing the risk of recurrent vascular events when compared to medical treatment. The benefit of TC PFO closure may be greater in patients with a substantial shunt.
See page 3336 for the editorial comment on this article (doi:10.1093/eurheartj/eht340)
Introduction
Patent foramen ovale (PFO) is a common finding in healthy adults.1 In patients with cryptogenic stroke, its prevalence has been found to be higher (up to 66%) when compared with patients with stroke from known causes.1
This difference in prevalence led to investigation of transcatheter (TC) PFO closure as a possible means of secondary prevention of neurological events. A recent comparative meta-analysis of observational studies showed that TC PFO closure was associated with an 84% reduction in the rate of recurrent neurological events when compared with medical management.2 However, results from three recently published randomized controlled trials (RCTs), failed to show a significant benefit of TC PFO closure over medical therapy.3–5
One of the main limitations of all three RCTs3–5 was the small number of events during the follow-up, raising the possibility of a ‘type 2 error’ (failure to detect a true difference between treatments due to lack of Power). With this in mind, we performed a systematic review and meta-analysis of the available RCT to evaluate possible benefits of TC PFO closure when compared with medical therapy for the prevention of recurrent neurological events.
Methods
Search strategy and selection criteria
We searched, for studies reporting death, transient ischaemic attack (TIA), and stroke in adult patients with previous TIA and/or stroke and the diagnosis of PFO. Medline, Pubmed, Embase, and Cochrane electronic databases were reviewed using the search terms ‘patent foramen ovale’, ‘PFO’, ‘stroke’, ‘percutaneous closure’ and ‘transcatheter closure’, with no language restrictions, from their inception to April 2013. Eligible studies were limited to RCT with at least 30 days follow-up. We supplemented the search with references from selected articles, and correspondence with other investigators.
Two investigators (P.R.M. and J.P.) independently assessed the studies for eligibility and their agreement was tested. Inclusion criteria were: (i) studies including adult patients (>18 years of age) with a history of TIA and/or stroke with a diagnosis of PFO; (ii) RCT of TC PFO closure compared with medical treatment (antiplatelet and/or anticoagulation); (iii) reported risk estimates and/or number of events for death from any cause, neurological event, TIA and/or stroke (fatal and non-fatal), and peripheral embolization; (iv) follow-up of at least 30 days following randomization. Standardized data collection sheets were used to gather data on age, sex, cardiovascular risk factors, use of antiplatelet or anticoagulation therapy, success rate for TC PFO closure, complications related to TC PFO closure (minor and major complications), presence or absence of atrial septal aneurysm, and shunt magnitude if reported in the studies. Agreement was also calculated for data extraction.
Two investigators (P.R.M. and J.P.) independently assessed the quality of the manuscripts and their agreement was tested. We used the Jadad score to assess the quality of trials that accounts for randomization (0, 1, or 2 points), blinding (single, double, or triple = 0, 1, 2 points) and lost to follow-up (0, 1 points). A score of ≥3 is deemed to be very good.6–9 When disagreement between investigators was present, a third investigator (A.R.C.) abstracted that specific data, and a meeting between the three investigators was taken place to reach agreement.
Statistical analysis
We abstracted risk estimates (relative risks, odds ratios, and hazard ratios with 95% confidence intervals) and total numbers of adverse events, comparing TC PFO closure to medical therapy. Agreement for inclusion of the studies, data extraction, and quality assessment was tested by calculating Cohen's kappa statistic.
Data for the main outcomes were pooled with the generic inverse variance method (logarithm of the risk estimate and its standard error) using fixed and random effect models (that accounts for the heterogeneity of the treatment effect across studies), yielding similar parameter estimates. We performed a meta-analysis and obtained pooled risk estimates for the development of TIA and/or stroke, and stroke alone for the TC PFO closure group when compared with medical treatment. For the combined outcome (death, TIA and/or stroke, peripheral embolism) pooled risk estimates for intention-to-treat (all three studies), and per-protocol (two studies) analyses were estimated, as these data were available.
Combining the three trials, we obtained an average for successful TC PFO closure and minor and major complications related to the procedure. Estimates for the development of new-onset atrial fibrillation between TC PFO closure vs. medical therapy were obtained by estimating odds ratios with 95% CI based on raw data reported in the trials.
Subgroup analyses were defined in advance to assess features that have been suggested as ‘high-risk PFO’ on the development of vascular outcomes,10–12 such as: age (younger vs. older group), presence or absence of atrial septal aneurysm, and a substantial shunt. All studies reported risk estimates as hazard ratios and 95% confidence intervals (which accounts for time-to-event). We tested for risk-subgroup interactions when risk estimates differed significantly between studies and interventions, including type of device (STARFlex vs. Amplatzer). Heterogeneity was tested with the χ2 test and inconsistency was quantified with the I2 statistic. The RevMan 4.2.8 software was used for analyses.13
Results
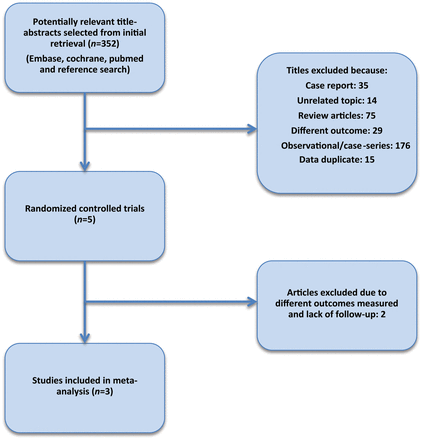
Of the 352 articles initially identified, three studies met the inclusion criteria.3–5 The agreement for inclusion of the studies was excellent (κ = 1.0). Figure 1 shows details of the study selection.
The three studies included, collected data between 2000 and 2009 in populations from North America, Canada, Europe, Brazil, and Australia (Table 1). Combining the three studies, a total of 2303 patients were randomized to TC PFO closure (n = 1150) or medical therapy (n = 1153). Exclusion criteria for each study can be found in the Supplementary material online,Supplementary Data.
Trials baseline characteristics
| Author . | Study acronym . | Enrolment . | Country . | Number of patients . | Mean follow-up (months) . | Lost to F/U . | Intervention group . | Medical therapy group . | Study conclusions . |
|---|---|---|---|---|---|---|---|---|---|
| Carroll et al. | RESPECT | 2003–11 multicentre, randomized | USA and Canada | 980 | 31 | Medical group 17.2% 83/481 | Amplatzer PFO occluder + aspirin and clopidogrel for 1 month followed by aspirin for at least 5 months | Aspirin 46.5% Coumadin 25.2% Clopidogrel 14% Aspirin + dipyridamole 8.1% Aspirin + clopidogrel 6.2% | No significant benefit of PFO closure for recurrent stroke prevention |
| Device group 9.2% 46/499 | |||||||||
| Meier et al. | PC | 2000–09 multicentre randomization by web-based system | 29 Centres in Europe, Canada, Brazil, and Australia | 414 | 49 | Medical group 15% 31/210 | Amplatzer PFO occluder + aspirin (5–6 months) and ticlopidine OR clopidogrel | Antiplatelet OR, AND coumadin (left at the discretion of treating physician) | No significant reduction in the risk of recurrent embolic events or death in the closure group, as compared with the medical therapy group |
| Device group 12% 24/204 | |||||||||
| Furlan et al. | CLOSURE I | 2003–08 multicentre, randomized | USA and Canada | 909 | 44 | Medical group 17% 77/462 | STARFlex + aspirin (2 years) and clopidogrel (6 months) | Aspirin, coumadin OR aspirin and coumadin (left at the discretion of treating physician) | No significant difference between closure with a percutaneous device plus antiplatelet therapy and medical therapy alone with respect to the prevention of recurrent stroke or TIA |
| Device group 5%, 24/447 |
| Author . | Study acronym . | Enrolment . | Country . | Number of patients . | Mean follow-up (months) . | Lost to F/U . | Intervention group . | Medical therapy group . | Study conclusions . |
|---|---|---|---|---|---|---|---|---|---|
| Carroll et al. | RESPECT | 2003–11 multicentre, randomized | USA and Canada | 980 | 31 | Medical group 17.2% 83/481 | Amplatzer PFO occluder + aspirin and clopidogrel for 1 month followed by aspirin for at least 5 months | Aspirin 46.5% Coumadin 25.2% Clopidogrel 14% Aspirin + dipyridamole 8.1% Aspirin + clopidogrel 6.2% | No significant benefit of PFO closure for recurrent stroke prevention |
| Device group 9.2% 46/499 | |||||||||
| Meier et al. | PC | 2000–09 multicentre randomization by web-based system | 29 Centres in Europe, Canada, Brazil, and Australia | 414 | 49 | Medical group 15% 31/210 | Amplatzer PFO occluder + aspirin (5–6 months) and ticlopidine OR clopidogrel | Antiplatelet OR, AND coumadin (left at the discretion of treating physician) | No significant reduction in the risk of recurrent embolic events or death in the closure group, as compared with the medical therapy group |
| Device group 12% 24/204 | |||||||||
| Furlan et al. | CLOSURE I | 2003–08 multicentre, randomized | USA and Canada | 909 | 44 | Medical group 17% 77/462 | STARFlex + aspirin (2 years) and clopidogrel (6 months) | Aspirin, coumadin OR aspirin and coumadin (left at the discretion of treating physician) | No significant difference between closure with a percutaneous device plus antiplatelet therapy and medical therapy alone with respect to the prevention of recurrent stroke or TIA |
| Device group 5%, 24/447 |
PFO, patent foramen ovale.
Trials baseline characteristics
| Author . | Study acronym . | Enrolment . | Country . | Number of patients . | Mean follow-up (months) . | Lost to F/U . | Intervention group . | Medical therapy group . | Study conclusions . |
|---|---|---|---|---|---|---|---|---|---|
| Carroll et al. | RESPECT | 2003–11 multicentre, randomized | USA and Canada | 980 | 31 | Medical group 17.2% 83/481 | Amplatzer PFO occluder + aspirin and clopidogrel for 1 month followed by aspirin for at least 5 months | Aspirin 46.5% Coumadin 25.2% Clopidogrel 14% Aspirin + dipyridamole 8.1% Aspirin + clopidogrel 6.2% | No significant benefit of PFO closure for recurrent stroke prevention |
| Device group 9.2% 46/499 | |||||||||
| Meier et al. | PC | 2000–09 multicentre randomization by web-based system | 29 Centres in Europe, Canada, Brazil, and Australia | 414 | 49 | Medical group 15% 31/210 | Amplatzer PFO occluder + aspirin (5–6 months) and ticlopidine OR clopidogrel | Antiplatelet OR, AND coumadin (left at the discretion of treating physician) | No significant reduction in the risk of recurrent embolic events or death in the closure group, as compared with the medical therapy group |
| Device group 12% 24/204 | |||||||||
| Furlan et al. | CLOSURE I | 2003–08 multicentre, randomized | USA and Canada | 909 | 44 | Medical group 17% 77/462 | STARFlex + aspirin (2 years) and clopidogrel (6 months) | Aspirin, coumadin OR aspirin and coumadin (left at the discretion of treating physician) | No significant difference between closure with a percutaneous device plus antiplatelet therapy and medical therapy alone with respect to the prevention of recurrent stroke or TIA |
| Device group 5%, 24/447 |
| Author . | Study acronym . | Enrolment . | Country . | Number of patients . | Mean follow-up (months) . | Lost to F/U . | Intervention group . | Medical therapy group . | Study conclusions . |
|---|---|---|---|---|---|---|---|---|---|
| Carroll et al. | RESPECT | 2003–11 multicentre, randomized | USA and Canada | 980 | 31 | Medical group 17.2% 83/481 | Amplatzer PFO occluder + aspirin and clopidogrel for 1 month followed by aspirin for at least 5 months | Aspirin 46.5% Coumadin 25.2% Clopidogrel 14% Aspirin + dipyridamole 8.1% Aspirin + clopidogrel 6.2% | No significant benefit of PFO closure for recurrent stroke prevention |
| Device group 9.2% 46/499 | |||||||||
| Meier et al. | PC | 2000–09 multicentre randomization by web-based system | 29 Centres in Europe, Canada, Brazil, and Australia | 414 | 49 | Medical group 15% 31/210 | Amplatzer PFO occluder + aspirin (5–6 months) and ticlopidine OR clopidogrel | Antiplatelet OR, AND coumadin (left at the discretion of treating physician) | No significant reduction in the risk of recurrent embolic events or death in the closure group, as compared with the medical therapy group |
| Device group 12% 24/204 | |||||||||
| Furlan et al. | CLOSURE I | 2003–08 multicentre, randomized | USA and Canada | 909 | 44 | Medical group 17% 77/462 | STARFlex + aspirin (2 years) and clopidogrel (6 months) | Aspirin, coumadin OR aspirin and coumadin (left at the discretion of treating physician) | No significant difference between closure with a percutaneous device plus antiplatelet therapy and medical therapy alone with respect to the prevention of recurrent stroke or TIA |
| Device group 5%, 24/447 |
PFO, patent foramen ovale.
Baseline characteristics, such as age, sex, and cardiovascular risk factors, were similar between treatment groups in all the studies (Table 2). Agreement for data inclusion was very good (κ = 0.80).
Baseline characteristics and risk factors
| Trial . | Number of patients . | Age (years) means ± SD . | Men (%) . | ASA (%) . | Hypertension (%) . | Hyperlipidaemia (%) . | Smoking (%) . | Diabetes (%) . | |
|---|---|---|---|---|---|---|---|---|---|
| RESPECT, n = 980 | Device group | 499 | 45.7 ± 9.7 | 268 (53.7) | 180 (36.1) | 158 (31.7) | 194 (38.9) | 75 (15) | 33 (6.6) |
| Medical therapy | 481 | 46.2 ± 10.0 | 268 (55.7) | 169 (35.1) | 150 (31.2) | 193 (40.1) | 55 (11.4) | 40 (8.3) | |
| PC, n = 414 | Device group | 204 | 44.3 ± 10.2 | 92 (45.1) | 47 (23.0) | 49 (24.0) | 50 (24.5) | 52 (25.5) | 5 (2.5) |
| Medical therapy | 210 | 44.6 ± 10.1 | 114 (54.3) | 51 (24.3) | 58 (27.6) | 62 (29.5) | 47 (22.4) | 6 (2.9) | |
| CLOSURE I, n = 909 | Device group | 447 | 46.3 ± 9.6 | 233 (52.1) | 168 (37.6) | 151 (33.8) | 212 (47.4) | 96 (21.5) | NR |
| Medical therapy | 462 | 45.7 ± 9.1 | 238 (51.5) | 165 (35.7) | 131 (28.4) | 189 (40.9) | 104 (22.6) | NR | |
| Trial . | Number of patients . | Age (years) means ± SD . | Men (%) . | ASA (%) . | Hypertension (%) . | Hyperlipidaemia (%) . | Smoking (%) . | Diabetes (%) . | |
|---|---|---|---|---|---|---|---|---|---|
| RESPECT, n = 980 | Device group | 499 | 45.7 ± 9.7 | 268 (53.7) | 180 (36.1) | 158 (31.7) | 194 (38.9) | 75 (15) | 33 (6.6) |
| Medical therapy | 481 | 46.2 ± 10.0 | 268 (55.7) | 169 (35.1) | 150 (31.2) | 193 (40.1) | 55 (11.4) | 40 (8.3) | |
| PC, n = 414 | Device group | 204 | 44.3 ± 10.2 | 92 (45.1) | 47 (23.0) | 49 (24.0) | 50 (24.5) | 52 (25.5) | 5 (2.5) |
| Medical therapy | 210 | 44.6 ± 10.1 | 114 (54.3) | 51 (24.3) | 58 (27.6) | 62 (29.5) | 47 (22.4) | 6 (2.9) | |
| CLOSURE I, n = 909 | Device group | 447 | 46.3 ± 9.6 | 233 (52.1) | 168 (37.6) | 151 (33.8) | 212 (47.4) | 96 (21.5) | NR |
| Medical therapy | 462 | 45.7 ± 9.1 | 238 (51.5) | 165 (35.7) | 131 (28.4) | 189 (40.9) | 104 (22.6) | NR | |
ASA, atrial septal aneurysm.
Baseline characteristics and risk factors
| Trial . | Number of patients . | Age (years) means ± SD . | Men (%) . | ASA (%) . | Hypertension (%) . | Hyperlipidaemia (%) . | Smoking (%) . | Diabetes (%) . | |
|---|---|---|---|---|---|---|---|---|---|
| RESPECT, n = 980 | Device group | 499 | 45.7 ± 9.7 | 268 (53.7) | 180 (36.1) | 158 (31.7) | 194 (38.9) | 75 (15) | 33 (6.6) |
| Medical therapy | 481 | 46.2 ± 10.0 | 268 (55.7) | 169 (35.1) | 150 (31.2) | 193 (40.1) | 55 (11.4) | 40 (8.3) | |
| PC, n = 414 | Device group | 204 | 44.3 ± 10.2 | 92 (45.1) | 47 (23.0) | 49 (24.0) | 50 (24.5) | 52 (25.5) | 5 (2.5) |
| Medical therapy | 210 | 44.6 ± 10.1 | 114 (54.3) | 51 (24.3) | 58 (27.6) | 62 (29.5) | 47 (22.4) | 6 (2.9) | |
| CLOSURE I, n = 909 | Device group | 447 | 46.3 ± 9.6 | 233 (52.1) | 168 (37.6) | 151 (33.8) | 212 (47.4) | 96 (21.5) | NR |
| Medical therapy | 462 | 45.7 ± 9.1 | 238 (51.5) | 165 (35.7) | 131 (28.4) | 189 (40.9) | 104 (22.6) | NR | |
| Trial . | Number of patients . | Age (years) means ± SD . | Men (%) . | ASA (%) . | Hypertension (%) . | Hyperlipidaemia (%) . | Smoking (%) . | Diabetes (%) . | |
|---|---|---|---|---|---|---|---|---|---|
| RESPECT, n = 980 | Device group | 499 | 45.7 ± 9.7 | 268 (53.7) | 180 (36.1) | 158 (31.7) | 194 (38.9) | 75 (15) | 33 (6.6) |
| Medical therapy | 481 | 46.2 ± 10.0 | 268 (55.7) | 169 (35.1) | 150 (31.2) | 193 (40.1) | 55 (11.4) | 40 (8.3) | |
| PC, n = 414 | Device group | 204 | 44.3 ± 10.2 | 92 (45.1) | 47 (23.0) | 49 (24.0) | 50 (24.5) | 52 (25.5) | 5 (2.5) |
| Medical therapy | 210 | 44.6 ± 10.1 | 114 (54.3) | 51 (24.3) | 58 (27.6) | 62 (29.5) | 47 (22.4) | 6 (2.9) | |
| CLOSURE I, n = 909 | Device group | 447 | 46.3 ± 9.6 | 233 (52.1) | 168 (37.6) | 151 (33.8) | 212 (47.4) | 96 (21.5) | NR |
| Medical therapy | 462 | 45.7 ± 9.1 | 238 (51.5) | 165 (35.7) | 131 (28.4) | 189 (40.9) | 104 (22.6) | NR | |
ASA, atrial septal aneurysm.
The mean of follow-up was 3.45 years, and varied across studies (4.1 years for the PC trial, 2 years for the CLOSURE I trial, and 2.6 years for the RESPECT trial). However, risk estimates in all the studies were presented as hazard ratios, which accounts for time-to-event. The average lost to follow-up was 12%. The agreement for all variables of quality by the Jadad score (randomization, blinding, and lost to follow-up) was good (κ = 0.63), and there was excellent agreement (κ = 1.0) that all studies were of very good quality (≥3 on the Jadad score).
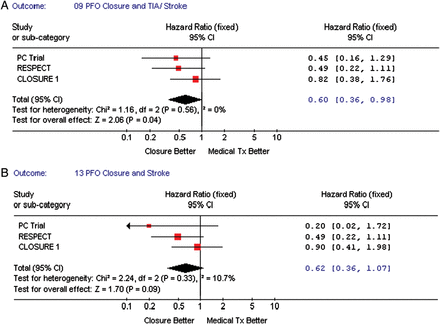
The primary outcome for the three studies was different (Table 3). Overall there were 95 vascular events in the three studies combined, 39 (3.39%) in the TC PFO closure group, and 56 (4.85%) in the medical treatment group. When focusing on TIA or stroke outcomes, a total of 98 events occurred in the three studies combined, 40 (3.47%) in the TC PFO closure group vs. 58 (5.03%) in the medical treatment group. For the primary outcome of our study, recurrent neurological events—stroke and/or TIA, there was a statistically significant benefit of TC PFO closure when compared with medical therapy, pooled HR = 0.59, 95% CI (0.36–0.97), P-value = 0.04 (Figure 2A). When assessing stroke alone, there was no benefit from the TC PFO closure when compared with medical therapy, pooled HR = 0.62, 95% CI (0.36–1.07), P-value = 0.09 (Figure 2B).
Outcomes for included trials
| . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval . | P-value . |
|---|---|---|---|---|---|---|
| RESPECT intention-to-treat | Non-fatal ischaemic stroke | 9/499 | 16/481 | 0.49 | 0.22–1.11 | 0.08 |
| RESPECT per protocol | 6/471 | 14/473 | 0.37 | 0.14–0.96 | 0.03 | |
| RESPECT as-treated | 5/474 | 16/484 | 0.27 | 0.1–0.75 | 0.007 | |
| PC | Composite: death, stroke, TIA or peripheral embolism | 7 (3.4) | 11 (5.2) | 0.63 | 0.24–1.62 | 0.34 |
| Death | 2 (1.0) | 0 | 5.2 | 0.25–107.61 | 0.24 | |
| Stroke | 1 (0.5) | 5 (2.4) | 0.2 | 0.02–1.72 | 0.14 | |
| TIA | 5 (2.5) | 7 (3.3) | 0.71 | 0.23–2.24 | 0.56 | |
| Composite stroke, TIA, peripheral embolism | 5 (2.5) | 11 (5.2) | 0.45 | 0.16–1.29 | 0.14 | |
| CLOSURE I intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 23 (5.5) | 29 (6.8) | 0.78 | 0.45–1.35 | 0.37 |
| Stroke | 12 (2.9) | 13 (3.1) | 0.9 | 0.41–1.98 | 0.79 | |
| TIA | 13 (3.1) | 17 (4.1) | 0.75 | 0.36–1.55 | 0.44 | |
| CLOSURE I modified intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/400 (5.6) | 29/451 (6.9) | 0.78 | 0.44–1.35 | 0.37 |
| Stroke | 12/400 (3.1) | 13/451 (3.1) | 0.94 | 0.43–2.07 | 0.88 | |
| TIA | 12/400 (3.0) | 17/451 (4.2) | 0.72 | 0.34–1.51 | 0.38 | |
| CLOSURE I per protocol | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/378 | 29/375 | 0.74 | 0.42–1.29 | 0.28 |
| Stroke | 12/378 | 13/375 | 0.91 | 0.41–1.99 | 0.8 | |
| TIA | 12/378 | 17/375 | 0.68 | 0.33–1.43 | 0.31 |
| . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval . | P-value . |
|---|---|---|---|---|---|---|
| RESPECT intention-to-treat | Non-fatal ischaemic stroke | 9/499 | 16/481 | 0.49 | 0.22–1.11 | 0.08 |
| RESPECT per protocol | 6/471 | 14/473 | 0.37 | 0.14–0.96 | 0.03 | |
| RESPECT as-treated | 5/474 | 16/484 | 0.27 | 0.1–0.75 | 0.007 | |
| PC | Composite: death, stroke, TIA or peripheral embolism | 7 (3.4) | 11 (5.2) | 0.63 | 0.24–1.62 | 0.34 |
| Death | 2 (1.0) | 0 | 5.2 | 0.25–107.61 | 0.24 | |
| Stroke | 1 (0.5) | 5 (2.4) | 0.2 | 0.02–1.72 | 0.14 | |
| TIA | 5 (2.5) | 7 (3.3) | 0.71 | 0.23–2.24 | 0.56 | |
| Composite stroke, TIA, peripheral embolism | 5 (2.5) | 11 (5.2) | 0.45 | 0.16–1.29 | 0.14 | |
| CLOSURE I intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 23 (5.5) | 29 (6.8) | 0.78 | 0.45–1.35 | 0.37 |
| Stroke | 12 (2.9) | 13 (3.1) | 0.9 | 0.41–1.98 | 0.79 | |
| TIA | 13 (3.1) | 17 (4.1) | 0.75 | 0.36–1.55 | 0.44 | |
| CLOSURE I modified intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/400 (5.6) | 29/451 (6.9) | 0.78 | 0.44–1.35 | 0.37 |
| Stroke | 12/400 (3.1) | 13/451 (3.1) | 0.94 | 0.43–2.07 | 0.88 | |
| TIA | 12/400 (3.0) | 17/451 (4.2) | 0.72 | 0.34–1.51 | 0.38 | |
| CLOSURE I per protocol | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/378 | 29/375 | 0.74 | 0.42–1.29 | 0.28 |
| Stroke | 12/378 | 13/375 | 0.91 | 0.41–1.99 | 0.8 | |
| TIA | 12/378 | 17/375 | 0.68 | 0.33–1.43 | 0.31 |
TIA, transient ischaemic attack.
Outcomes for included trials
| . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval . | P-value . |
|---|---|---|---|---|---|---|
| RESPECT intention-to-treat | Non-fatal ischaemic stroke | 9/499 | 16/481 | 0.49 | 0.22–1.11 | 0.08 |
| RESPECT per protocol | 6/471 | 14/473 | 0.37 | 0.14–0.96 | 0.03 | |
| RESPECT as-treated | 5/474 | 16/484 | 0.27 | 0.1–0.75 | 0.007 | |
| PC | Composite: death, stroke, TIA or peripheral embolism | 7 (3.4) | 11 (5.2) | 0.63 | 0.24–1.62 | 0.34 |
| Death | 2 (1.0) | 0 | 5.2 | 0.25–107.61 | 0.24 | |
| Stroke | 1 (0.5) | 5 (2.4) | 0.2 | 0.02–1.72 | 0.14 | |
| TIA | 5 (2.5) | 7 (3.3) | 0.71 | 0.23–2.24 | 0.56 | |
| Composite stroke, TIA, peripheral embolism | 5 (2.5) | 11 (5.2) | 0.45 | 0.16–1.29 | 0.14 | |
| CLOSURE I intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 23 (5.5) | 29 (6.8) | 0.78 | 0.45–1.35 | 0.37 |
| Stroke | 12 (2.9) | 13 (3.1) | 0.9 | 0.41–1.98 | 0.79 | |
| TIA | 13 (3.1) | 17 (4.1) | 0.75 | 0.36–1.55 | 0.44 | |
| CLOSURE I modified intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/400 (5.6) | 29/451 (6.9) | 0.78 | 0.44–1.35 | 0.37 |
| Stroke | 12/400 (3.1) | 13/451 (3.1) | 0.94 | 0.43–2.07 | 0.88 | |
| TIA | 12/400 (3.0) | 17/451 (4.2) | 0.72 | 0.34–1.51 | 0.38 | |
| CLOSURE I per protocol | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/378 | 29/375 | 0.74 | 0.42–1.29 | 0.28 |
| Stroke | 12/378 | 13/375 | 0.91 | 0.41–1.99 | 0.8 | |
| TIA | 12/378 | 17/375 | 0.68 | 0.33–1.43 | 0.31 |
| . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval . | P-value . |
|---|---|---|---|---|---|---|
| RESPECT intention-to-treat | Non-fatal ischaemic stroke | 9/499 | 16/481 | 0.49 | 0.22–1.11 | 0.08 |
| RESPECT per protocol | 6/471 | 14/473 | 0.37 | 0.14–0.96 | 0.03 | |
| RESPECT as-treated | 5/474 | 16/484 | 0.27 | 0.1–0.75 | 0.007 | |
| PC | Composite: death, stroke, TIA or peripheral embolism | 7 (3.4) | 11 (5.2) | 0.63 | 0.24–1.62 | 0.34 |
| Death | 2 (1.0) | 0 | 5.2 | 0.25–107.61 | 0.24 | |
| Stroke | 1 (0.5) | 5 (2.4) | 0.2 | 0.02–1.72 | 0.14 | |
| TIA | 5 (2.5) | 7 (3.3) | 0.71 | 0.23–2.24 | 0.56 | |
| Composite stroke, TIA, peripheral embolism | 5 (2.5) | 11 (5.2) | 0.45 | 0.16–1.29 | 0.14 | |
| CLOSURE I intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 23 (5.5) | 29 (6.8) | 0.78 | 0.45–1.35 | 0.37 |
| Stroke | 12 (2.9) | 13 (3.1) | 0.9 | 0.41–1.98 | 0.79 | |
| TIA | 13 (3.1) | 17 (4.1) | 0.75 | 0.36–1.55 | 0.44 | |
| CLOSURE I modified intention-to-treat | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/400 (5.6) | 29/451 (6.9) | 0.78 | 0.44–1.35 | 0.37 |
| Stroke | 12/400 (3.1) | 13/451 (3.1) | 0.94 | 0.43–2.07 | 0.88 | |
| TIA | 12/400 (3.0) | 17/451 (4.2) | 0.72 | 0.34–1.51 | 0.38 | |
| CLOSURE I per protocol | Composite: death from any cause during first 30 days, death from neurological causes between 31 days and 2 years, stroke, and TIA | 22/378 | 29/375 | 0.74 | 0.42–1.29 | 0.28 |
| Stroke | 12/378 | 13/375 | 0.91 | 0.41–1.99 | 0.8 | |
| TIA | 12/378 | 17/375 | 0.68 | 0.33–1.43 | 0.31 |
TIA, transient ischaemic attack.
(A) The forest plot of randomized controlled trial comparing vascular outcomes (stroke/transient ischaemic attack) between transcatheter patent foramen ovale closure vs. medical treatment (intention-to-treat). (B) The forest plot of the randomized controlled trial comparing vascular outcomes (stroke) between transcatheter patent foramen ovale closure vs. medical treatment (intention-to-treat).
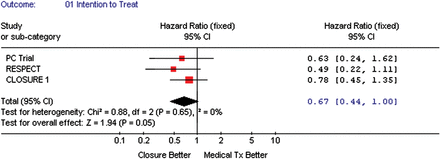
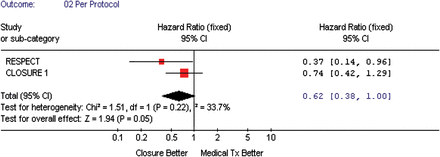
All three studies presented a composite outcome (death, recurrent neurological events, and peripheral embolism) based on intention-to-treat analyses (Figure 3), showing a possible benefit of the TC PFO closure that was borderline statistically significant when compared with medical treatment, pooled HR = 0.67, 95% CI (0.44–1.00), P-value = 0.05. Two studies presented their analyses on a per-protocol basis (Figure 4), which again showed a possible benefit of the TC PFO closure that was borderline statistically significant when compared with medical therapy, pooled HR = 0.62, 95% CI (0.38–1.00), P-value = 0.05.
The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment (intention-to-treat).
The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment (per protocol).
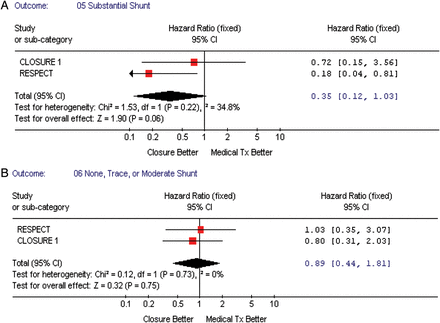
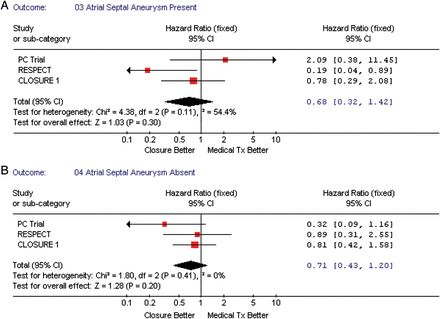
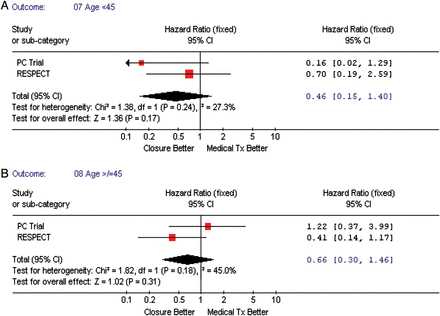
We performed subgroup analyses to assess which ‘high-risk PFO’ characteristics were associated with vascular events, namely—the presence of an atrial septal aneurysm (all three studies), shunt magnitude at baseline (two studies), and age <45 vs. >45 years (two studies). Figure 5A and B shows that subjects with a significant shunt (substantial vs. trace, none, or moderate) had a tendency towards decreased vascular events in patients when randomized to the TC PFO closure when compared with medical therapy, pooled HR = 0.35, 95% CI (0.12–1.03), P-value = 0.06. However, when testing for interaction between risk estimates by type of intervention regarding shunt magnitude, the P-value was non-significant (P = 0.15). Both, the presence or absence of atrial septal aneurysm (Figure 6A and B), and age (≥45 vs. <45 years, Figure 7A and B) were not associated with an increased incidence of vascular events.
(A) The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment by shunt magnitude (substantial). (B) The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment by shunt magnitude (trace, none, or moderate).
(A) The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment by the presence of atrial septal aneurysm. (B) The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment by the absence of atrial septal aneurysm.
(A) The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment in patients >45 years. (B) The forest plot of randomized controlled trial comparing composite outcome (death/vascular events) between transcatheter patent foramen ovale closure vs. medical treatment in patients <45 years.
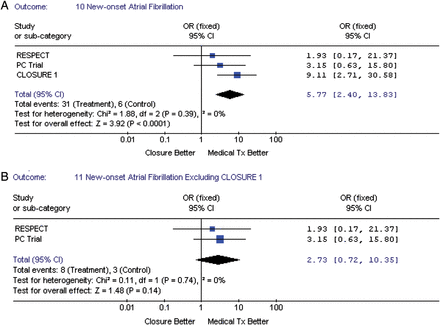
Device implantation was successful in 93.8% on average (Table 1), being lowest with the STARFlex device in the CLOSURE I trial (89.4%). On average, there were 13 (1.13%) vascular complications, and there were two cases (0.17%) of cardiac perforation. There were no fatal events related to the TC PFO closure (Table 4). New-onset atrial fibrillation was significantly higher in the TC PFO group (Figure 8A) when compared with medical therapy 31 (2.7) vs. 6 (0.5%), respectively, pooled OR = 5.77, 95% CI (2.4–13.83), P < 0.001. When stratifying by type of device (excluding STARFlex device), the Amplatzer device (Figure 8B) had a non-significant increased risk for developing new-onset atrial fibrillation: 8 (1.1) vs. 3 (0.4%), respectively, pooled OR = 2.73, 95% CI (0.72–10.33), P = 0.14, though, the test for interaction between risk estimates by closure device was not significant (P = 0.18).
Adverse events for included trials
| Trial . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval (95% CI) . | P-value . |
|---|---|---|---|---|---|---|
| PC trial | New-onset atrial fibrillation | 6 (2.9) Two transient, 2 required pharmacological cardioversion, 1 required electrical cardioversion, and 1 sustained AF | 2 (1) | 3.15 | 0.64–15.6 | 0.16 |
| Myocardial infarction | 2 (1) | 1 (0.5) | 2.04 | 0.19–22.5 | 0.62 | |
| Hospital admission related to patent foramen ovale | 13 (6.4) | 13 (6.3) | 1.02 | 0.48–2.21 | 0.95 | |
| Bleeding | 8 (3.9) | 12 (5.7) | 0.66 | 0.27–1.62 | 0.40 | |
| Vascular procedural complication | 2 (1) | N/A | N/A | N/A | N/A | |
| CLOSURE 1 | New-onset atrial fibrillation | 23 (5.7) Only 14 during the initial 30 days of follow-up, it was transient in 17 patients and persistent in 6 patients | 3 (0.7) | N/A | N/A | <0.001 |
| Major bleeding episode | 10/378 (2.6) | 4/374 (1.1) | N/A | N/A | 0.11 | |
| Death other than endpoint | 2 (0.5) | 4 (0.9) | N/A | N/A | 0.51 | |
| Nervous system disorder | 6 (1.5) | 16 (3.5) | N/A | N/A | 0.15 | |
| Vascular procedural complication | 8 (1.7) | N/A | N/A | N/A | N/A | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | |||
| RESPECT | New-onset atrial fibrillation | (3) | (1.5) | N/A | N/A | N/A |
| Pulmonary embolism | 6 (1.2) | 1 (0.2) | N/A | N/A | 0.12 | |
| Major bleeding episode | 8 (1.6) | 9 (1.9) | N/A | N/A | 0.81 | |
| Vascular procedural complication | 3 (0.6) | 0 | N/A | N/A | 0.124 | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | N/A | 0.124 |
| Trial . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval (95% CI) . | P-value . |
|---|---|---|---|---|---|---|
| PC trial | New-onset atrial fibrillation | 6 (2.9) Two transient, 2 required pharmacological cardioversion, 1 required electrical cardioversion, and 1 sustained AF | 2 (1) | 3.15 | 0.64–15.6 | 0.16 |
| Myocardial infarction | 2 (1) | 1 (0.5) | 2.04 | 0.19–22.5 | 0.62 | |
| Hospital admission related to patent foramen ovale | 13 (6.4) | 13 (6.3) | 1.02 | 0.48–2.21 | 0.95 | |
| Bleeding | 8 (3.9) | 12 (5.7) | 0.66 | 0.27–1.62 | 0.40 | |
| Vascular procedural complication | 2 (1) | N/A | N/A | N/A | N/A | |
| CLOSURE 1 | New-onset atrial fibrillation | 23 (5.7) Only 14 during the initial 30 days of follow-up, it was transient in 17 patients and persistent in 6 patients | 3 (0.7) | N/A | N/A | <0.001 |
| Major bleeding episode | 10/378 (2.6) | 4/374 (1.1) | N/A | N/A | 0.11 | |
| Death other than endpoint | 2 (0.5) | 4 (0.9) | N/A | N/A | 0.51 | |
| Nervous system disorder | 6 (1.5) | 16 (3.5) | N/A | N/A | 0.15 | |
| Vascular procedural complication | 8 (1.7) | N/A | N/A | N/A | N/A | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | |||
| RESPECT | New-onset atrial fibrillation | (3) | (1.5) | N/A | N/A | N/A |
| Pulmonary embolism | 6 (1.2) | 1 (0.2) | N/A | N/A | 0.12 | |
| Major bleeding episode | 8 (1.6) | 9 (1.9) | N/A | N/A | 0.81 | |
| Vascular procedural complication | 3 (0.6) | 0 | N/A | N/A | 0.124 | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | N/A | 0.124 |
Adverse events for included trials
| Trial . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval (95% CI) . | P-value . |
|---|---|---|---|---|---|---|
| PC trial | New-onset atrial fibrillation | 6 (2.9) Two transient, 2 required pharmacological cardioversion, 1 required electrical cardioversion, and 1 sustained AF | 2 (1) | 3.15 | 0.64–15.6 | 0.16 |
| Myocardial infarction | 2 (1) | 1 (0.5) | 2.04 | 0.19–22.5 | 0.62 | |
| Hospital admission related to patent foramen ovale | 13 (6.4) | 13 (6.3) | 1.02 | 0.48–2.21 | 0.95 | |
| Bleeding | 8 (3.9) | 12 (5.7) | 0.66 | 0.27–1.62 | 0.40 | |
| Vascular procedural complication | 2 (1) | N/A | N/A | N/A | N/A | |
| CLOSURE 1 | New-onset atrial fibrillation | 23 (5.7) Only 14 during the initial 30 days of follow-up, it was transient in 17 patients and persistent in 6 patients | 3 (0.7) | N/A | N/A | <0.001 |
| Major bleeding episode | 10/378 (2.6) | 4/374 (1.1) | N/A | N/A | 0.11 | |
| Death other than endpoint | 2 (0.5) | 4 (0.9) | N/A | N/A | 0.51 | |
| Nervous system disorder | 6 (1.5) | 16 (3.5) | N/A | N/A | 0.15 | |
| Vascular procedural complication | 8 (1.7) | N/A | N/A | N/A | N/A | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | |||
| RESPECT | New-onset atrial fibrillation | (3) | (1.5) | N/A | N/A | N/A |
| Pulmonary embolism | 6 (1.2) | 1 (0.2) | N/A | N/A | 0.12 | |
| Major bleeding episode | 8 (1.6) | 9 (1.9) | N/A | N/A | 0.81 | |
| Vascular procedural complication | 3 (0.6) | 0 | N/A | N/A | 0.124 | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | N/A | 0.124 |
| Trial . | Events . | Intervention group (%) . | Medical therapy (%) . | Hazard ratio . | Confidence interval (95% CI) . | P-value . |
|---|---|---|---|---|---|---|
| PC trial | New-onset atrial fibrillation | 6 (2.9) Two transient, 2 required pharmacological cardioversion, 1 required electrical cardioversion, and 1 sustained AF | 2 (1) | 3.15 | 0.64–15.6 | 0.16 |
| Myocardial infarction | 2 (1) | 1 (0.5) | 2.04 | 0.19–22.5 | 0.62 | |
| Hospital admission related to patent foramen ovale | 13 (6.4) | 13 (6.3) | 1.02 | 0.48–2.21 | 0.95 | |
| Bleeding | 8 (3.9) | 12 (5.7) | 0.66 | 0.27–1.62 | 0.40 | |
| Vascular procedural complication | 2 (1) | N/A | N/A | N/A | N/A | |
| CLOSURE 1 | New-onset atrial fibrillation | 23 (5.7) Only 14 during the initial 30 days of follow-up, it was transient in 17 patients and persistent in 6 patients | 3 (0.7) | N/A | N/A | <0.001 |
| Major bleeding episode | 10/378 (2.6) | 4/374 (1.1) | N/A | N/A | 0.11 | |
| Death other than endpoint | 2 (0.5) | 4 (0.9) | N/A | N/A | 0.51 | |
| Nervous system disorder | 6 (1.5) | 16 (3.5) | N/A | N/A | 0.15 | |
| Vascular procedural complication | 8 (1.7) | N/A | N/A | N/A | N/A | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | |||
| RESPECT | New-onset atrial fibrillation | (3) | (1.5) | N/A | N/A | N/A |
| Pulmonary embolism | 6 (1.2) | 1 (0.2) | N/A | N/A | 0.12 | |
| Major bleeding episode | 8 (1.6) | 9 (1.9) | N/A | N/A | 0.81 | |
| Vascular procedural complication | 3 (0.6) | 0 | N/A | N/A | 0.124 | |
| Cardiac perforation | 1 (0.2) | 0 | N/A | N/A | 0.124 |
(A) The forest plot of randomized controlled trial comparing new-onset atrial fibrillation between transcatheter patent foramen ovale closure vs. medical treatment. (B) The forest plot of randomized controlled trial comparing new-onset atrial fibrillation between transcatheter patent foramen ovale closure vs. medical treatment excluding STARFLEX closure device.
Heterogeneity in the analyses performed was low (0% in the intention-to-treat analyses for TIA and/or stroke and combined outcomes), except for analyses for the presence of atrial septal aneurysm (I2 = 54.4%), and age >45 years (I2 = 45%) groups.
Discussion
In this meta-analysis of RCT, we report a possible benefit from the TC PFO closure, when compared with medical therapy, for prevention of recurrent vascular events. In pooled analyses, there was a statistically significant 41% risk reduction in recurrent TIA or stroke. In addition, there was a 33% risk reduction in the risk for the composite outcome of death, neurological events, and peripheral embolism based on intention-to-treat analyses. However, when assessing for stroke prevention alone (excluding TIAs), TC PFO closure did not show a statistically significant benefit. These results support the notion that previous RCTs were underpowered.14
A recent meta-analysis of 48 observational studies showed an incidence of recurrent neurological events per year of 0.8% for the TC PFO closure group vs. 5% for the medical therapy group, which translates to 2.8% neurological events in the TC PFO group and 17.5% in the medical therapy group at 3.5 years of follow-up (14.7% absolute risk reduction).2 In contrast, our pooled results found an incidence of neurological events of 3.47% in the TC PFO vs. 5.03% in the medical group (1.56% absolute risk reduction) at a similar average follow-up (3.45 years). This important difference in absolute risk reduction raises the possibility of significant selection bias in these observational studies, in addition to all the potential biases inherent in non-randomized studies. Several possible reasons for fewer events among device-treated patients in the observational studies have been put forth.14,15 Among the most important are that type of treatment was based on physician preference, and the interventional therapy was being offered using off-label devices for septal defects, resulting on enrolment difficulties, and by unclear reasons, vascular events at follow-up have been lower than expected.14,15
In our analyses, we also explored features that have been suggested as ‘high-risk PFO’ in patients with cryptogenic stroke, such as young age, presence of atrial septal aneurysm, and shunt magnitude.10–12 In our analyses, the rate of recurrent neurological events in younger patients compared with older patients (<45 vs. ≥45 years) was not significantly different in the TC PFO closure group when compared with the medical group. In addition, the presence of an atrial septal aneurysm was not identified as a high-risk feature of future vascular events neither in the TC PFO nor medical therapy groups, but it is important to mention that these results had the greatest heterogeneity (54.4%), suggesting important differences between studies that could not be accounted in our analyses.
An important finding in our analyses is that patients with a ‘substantial shunt’ appear to have better outcomes with device closure; however, the results did not reach statistical significance (P = 0.06). We could not explore this hypothesis further, as the PC trial did not report their findings stratified by shunt magnitude. Ideally, collaboration from the investigators conducting these trials can result in joining individual data to further explore this finding.
Currently, there are no studies proving benefit of a particular class of medications for secondary prevention of cryptogenic stroke, and both antiplatelets and/or anticoagulation are deemed reasonable options.16,17 It is important to mention that the use of antiplatelet and/or anticoagulation therapy in these studies was at discretion of the treating physician. Because of this, and low event rate, no meaningful comparisons based on type of medication vs. TC PFO were possible.
Since atrial fibrillation is a major risk factor for stroke, all three trials excluded patients with this condition. Interestingly, in our analysis we found a small, but statistically significant, increased risk of developing new-onset atrial fibrillation with the TC PFO closure group when compared with medical therapy (2.7 vs. 0.5%, OR = 5.7 P < 0.0001). Despite this increased risk of new-onset atrial fibrillation, the TC PFO closure was still significantly associated with lower risk of TIA and/or stroke in our analyses. Furthermore, excluding CLOSURE I population from the analyses, TC PFO closure using the Amplatzer device, failed to demonstrate a statistically significant increased risk for the development of new-onset atrial fibrillation when compared with the medical therapy group. These findings are consistent with a recently reported meta-analysis of observational studies showing that STARFlex or CardiolSEAL, but not the Amplatzer device were associated with an increased risk of developing new-onset atrial fibrillation.18 Nonetheless, the impact of the potential increased risk for developing new-onset fibrillation in the TC PFO group, and its potential role in the development of neurological events should be further explored by future analyses, including individual data from these three trials where adjustment of this variable in their outcomes would be possible.
Regarding success of the TC PFO, the analyses show that it can be achieved at a high rate (∼94%), with low complications rates, and with no reported deaths related to the procedure itself. This becomes highly relevant as the medical treatment for cryptogenic stroke associated with PFO may include dual antiplatelet therapy and/or warfarin with their known potential risks.16,17
Strengths of our study include: (i) we followed rigorously the recommended guidelines for reporting and conducting meta-analysis of RCT,7–9 (ii) the use of proper quality assessment of the studies by a previously well-validated scale (Jadad),8 and (iii) all of our studies were of high quality with scores ≥3 points on the Jadad scale. In addition, all three populations had similar baseline characteristics, which make them suitable for meta-analysis (heterogeneity = 0% in the intention-to-treat and TIA/stroke analyses).
The major limitation of our study is that we lacked individual-level data for analyses. Given the similar populations studied, the similar study methodologies and the absence of heterogeneity in most of our analyses, we believe our results are robust. However, individual data are needed, not only to support the current findings, but also to clarify the role of ‘high-risk PFO features’, in the development of vascular events. Such data might also shed light on, possible impact of device-related new-onset atrial fibrillation on future neurological events. Another limitation is that the medical therapy group in all three trials did not receive a standardized therapy (antiplatelet and/or anticoagulant), but rather was left upon the physicians discretion, furthermore, the antiplatelet therapy in the intervention group differ between the three trials, making the interpretation of the data difficult. In addition, whether the benefit of the TC PFO closure will persist at long term is unclear as none of the studies had follow-up longer than 5 years. Finally, publication bias is unlikely in our study, as all three RCTs included had negative results.
In summary, this meta-analysis of RCT is the first study to show a possible benefit from the TC PFO closure for prevention of recurrent neurological events in patients with cryptogenic stroke when compared with medical therapy. This finding has the potential to affect clinical practice. Until further analyses with individual-level data are available or currently undergoing RCT with similar outcomes and appropriate follow-up are published,19,20 these results might be the best evidence of significant benefit from the TC PFO closure.
Conclusions
In patients with cryptogenic stroke, when compared with medical treatment, the TC PFO closure may be beneficial in reducing the risk of recurrent vascular events. The benefit of the TC PFO closure may be greater in patients with large degree of shunt, but these findings need to be confirmed in further studies. Finally, it appears that the TC PFO closure increases the risk of new-onset atrial fibrillation and might be associated with the type of device. Despite this increase in new-onset atrial fibrillation, the TC PFO closure showed a possible benefit in reducing the incidence of recurrent neurological events.
Conflicts of interest: none declared.
Acknowledgements
P.R.-M. contributed: design of the study, data extraction, and writing the manuscript. I.F.P. contributed: design of the study, and writing the manuscript. P.J. contributed: data extraction and writing the manuscript. C.F.W. contributed: writing the manuscript. L.D.M.: design of the study, and writing the manuscript. A.R.-C.: design of the study, data extraction, statistical analyses, and writing the manuscript. Special thanks to Dr Gregg Pressman (cardiologist) and Dr Ferdinando Buonanno (neurologist) for providing valuable insight during the preparation of the manuscript. We would like to thank all the investigators and personnel in charge of designing and completing the CLOSURE I, PC trial and RESPECT.




Comments
We appreciate the comments of Drs. Nagaraja and Eslick regarding our meta-analysis with reference to PFO closure. This letter, again, brings attention to the issue of PFO closure as an alternative approach for secondary prevention of cryptogenic stroke.
When we initially reviewed the results of the three trials that were included in our study(1,2,3), and identified potential issues that could explain the negative results. Among the most important were that all three were underpowered for their main endpoint, and they included several patients with a relatively small PFO, which might not represent the type of PFO that would most benefit from transcatheter closure (4).
We believe there are explanations for our results that are different compared to other studies (5,6,7,8,9,10,11). First, in our analyses for the main outcomes, we used pooled analyses using the generic inverse variance method, thus allowing us to obtain hazard ratios rather than odd ratios. This becomes very important due to the fact that despite odds ratios being "more robust" they have a lower statistical power, an issue related to all the included trials. This statistical fact may be the reason that the p-values we obtained are significant compared to other published meta-analyses.
In addition, as noted by Drs. Nagaraja and Eslick, we performed different sub-analyses in our study. Our main goal was to obtain results for the main outcomes as pre-established in the original trials. We attempted sub-analyses for stroke alone as we thought that this would be the most clinically relevant outcome for this condition. However, we do agree with Nagaraja and Eslick that if we had more events in these trials, other analyses for other variables would be important.
As acknowledged in our study, we are fully aware that meta-analyses have several limitations. In our case, not having raw data for analysis is a limitation. However, we also believe that meta-analyses may be valuable when trying to assess best practice regarding a clinical issue, and their results must be seriously considered. For example, Bravata et al. in their now classic meta-analysis, established the advantages and disadvantages of surgical versus percutaneous revascularization (12). This study serves as a fundation for many discussions regarding the benefits of each approach.
We also agree that a better understanding of TC PFO closure is needed, and ongoing studies such as (CLOSE, REDUCE, DEFENSE-PFO) will help to answer these questions.
With regards to the funding of the three RCTs, we know that it is very difficult to undertake a study of this magnitude with an expensive intervention without the contribution of industry. We place our trust in the ethical standards of investigators and the sponsors supporting these trials financially.
Pablo Rengifo-Moreno, MD, Christian F. Witzke, MD, D. Lynn Morris, MD, and Abel Romero-Corral, MD, MSc.
References
1. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366(11):991-9.
2. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368(12):1092-100.
3. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Juni P. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368(12):1083-91.
4. Steiner MM, Di Tullio MR, Rundek T, Gan R, Chen X, Liguori C, Brainin M, Homma S, Sacco RL. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke 1998;29:944-948.
5.Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is Transcatheter Closure Better than Medical Therapy for Cryptogenic Stroke with Patent Foramen Ovale? A Meta-analysis of Randomised Trials. Heart Lung Circ 2013.
6.Wolfrum M, Froehlich GM, Knapp G, Casaubon LK, Dinicolantonio JJ, Lansky AJ, Meier P. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2013.
7.Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: A meta-analysis of randomized controlled trials. Int J Cardiol 2013.
8.Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs. medical therapy in cryptogenic stroke or transient ischemic attack: A systematic review and meta-analysis. Int J Cardiol 2013.
9.Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013.
10.Kitsios GD, Thaler DE, Kent DM. Potentially Large yet Uncertain Benefits: A Meta-analysis of Patent Foramen Ovale Closure Trials. Stroke 2013;44(9):2640-2643.
11.Hakeem A, Marmagkiolis K, Hacioglu Y, Uretsky BF, Gundogdu B, Leesar M, Bailey SR, Cilingiroglu M. Safety and efficacy of device closure for patent foramen ovale for secondary prevention of neurological events: Comprehensive systematic review and meta-analysis of randomized controlled trials. Cardiovasc Revasc Med 2013.
12. Bravata DM, Gienger AL, McDonald KM, et al. Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann Intern Med. 2007;147: 703-16.
Conflict of Interest:
None declared
Sir,
With the greatest interest we have read the recent review by P. Rengifo-Moreno1 and co-authors on secondary prevention of cryptogenic stroke with a patent foramen ovale. The ideal approach for secondary prevention of cryptogenic stroke with a patent foramen ovale is uncertain. Astoundingly, in the last few months nine meta-analyses have been published regarding secondary prevention of cryptogenic stroke with a patent foramen ovale.1-9 Three articles published in the New England Journal of Medicine (CLOSURE 1 trial,10 The RESPECT trial11 and the PC-Trial12) were included in these meta-analyses. The most vital issue is that all three randomized clinical trials had negative outcomes and four of the subsequent meta-analyses2-5 conclude that there is no advantage.
Rengifo-Moreno et al1 established an advantage with closure in an intention-to-treat analysis (odd ratio (OR): 0.67; 95% CI: 0.44-1.00), which was maintained in a per-protocol analysis of 2 of the studies (OR: 0.62; 95% CI: 0.38-1.00) when the composite outcome of death, recurrent neurological events, and peripheral embolism were combined from the three trials. Conversely, the advantage vanished when looking at stroke alone (OR: 0.62; 95% CI: 0.36-1.07). They did not calculate for the TIA cohort nevertheless, PFO closure does not confer an benefit over medical therapy according to another meta-analysis (OR: 0.77, 95% CI: 0.41–1.43).2 The subgroup analyses showed that while the effectiveness of closure was not affected by the presence of “high-risk” characteristics such as atrial septal aneurysm or older age, patients with substantial shunt at baseline (studied in 2 trials) saw a trend toward less vascular events after PFO closure (OR: 0.35; 95% CI: 0.12-1.03), but was not statistically significant. The trend was no longer evident when testing for interaction between type of intervention and shunt magnitude. Furthermore, atrial fibrillation was higher in the closure group compared with the medical therapy group. Khan et al8 have published similar results however, did not perform subgroup analysis for stroke and TIA.
Given the substantial limitations of a meta-analysis in general, and the specific limitations of combining clinical trials using diverse devices13 and, importantly, very dissimilar medical management approaches in both the closure and medical arms, one would be hesitant to use such data to change clinical practice. It is important to note that all trials have been industry sponsored (CLOSURE I by NMT Medical, RESPECT and PC by St Jude Medical) and several of the investigators are ‘PFO closure supporters’. Without inferring that this has introduced a bias, it is important to consider, especially because the study designs were open label in all trials.
Ongoing PFO clinical trials (CLOSE, REDUCE, DEFENSE-PFO) will help us solve this continuing dilemma. Newer closure devices are under investigation (buttoned device, Angel wings, and bio-absorbable devices) which aim to reduce procedural complications and increase efficacy. Bigger randomized trials with extended follow-up are required to establish the advantage of percutaneous closure compared to medical therapy.
References
1. Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta- analysis of randomized controlled trials. Eur Heart J 2013.
2. Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is Transcatheter Closure Better than Medical Therapy for Cryptogenic Stroke with Patent Foramen Ovale? A Meta-analysis of Randomised Trials. Heart Lung Circ 2013.
3. Wolfrum M, Froehlich GM, Knapp G, Casaubon LK, Dinicolantonio JJ, Lansky AJ, Meier P. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2013.
4. Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: A meta-analysis of randomized controlled trials. Int J Cardiol 2013.
5. Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs. medical therapy in cryptogenic stroke or transient ischemic attack: A systematic review and meta-analysis. Int J Cardiol 2013.
6. Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013.
7. Kitsios GD, Thaler DE, Kent DM. Potentially Large yet Uncertain Benefits: A Meta-analysis of Patent Foramen Ovale Closure Trials. Stroke 2013;44(9):2640-2643.
8. Khan AR, Bin Abdulhak AA, Sheikh MA, Khan S, Erwin PJ, Tleyjeh I, Khuder S, Eltahawy EA. Device Closure of Patent Foramen Ovale Versus Medical Therapy in Cryptogenic Stroke: A Systematic Review and Meta- Analysis. JACC Cardiovasc Interv 2013.
9. Hakeem A, Marmagkiolis K, Hacioglu Y, Uretsky BF, Gundogdu B, Leesar M, Bailey SR, Cilingiroglu M. Safety and efficacy of device closure for patent foramen ovale for secondary prevention of neurological events: Comprehensive systematic review and meta-analysis of randomized controlled trials. Cardiovasc Revasc Med 2013.
10. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366(11):991-9.
11. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368(12):1092-100.
12. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Juni P. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368(12):1083-91.
13. Hornung M, Bertog SC, Franke J, Id D, Taaffe M, Wunderlich N, Vaskelyte L, Hofmann I, Sievert H. Long-term results of a randomized trial comparing three different devices for percutaneous closure of a patent foramen ovale. Eur Heart J 2013.
Conflict of Interest:
None declared