-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah C.W. Marott, Sune F. Nielsen, Marianne Benn, Børge G. Nordestgaard, Antihypertensive treatment and risk of atrial fibrillation: a nationwide study, European Heart Journal, Volume 35, Issue 18, 7 May 2014, Pages 1205–1214, https://doi.org/10.1093/eurheartj/eht507
Close - Share Icon Share
Abstract
To examine the associations between antihypertensive treatment with angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs), β-blockers, diuretics, or calcium-antagonists, and risk of atrial fibrillation. We examined these associations using the entire Danish population from 1995 through 2010.
Excluding medication used in atrial fibrillation, we matched individuals on ACEi monotherapy 1:1 with individuals on β-blocker (n = 48 658), diuretic (n = 69 630), calcium-antagonist (n = 57 646), and ARB monotherapy (n = 20 158). Likewise, individuals on ARB monotherapy were matched 1:1 with individuals on β-blocker (n = 20 566), diuretic (n = 20 832), calcium-antagonist (n = 20 232), and ACEi monotherapy (n = 20 158). All were free of atrial fibrillation and of predisposing diseases like heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline and none received any other antihypertensive medication. We studied risk of atrial fibrillation, and used risk of stroke, influenced by lowering blood pressure rather than renin-angiotensin system blockade per se, as an indicator of the importance of blood pressure lowering per se. Hazard ratios of atrial fibrillation for ACEi and ARB monotherapy were 0.12 (95% CI: 0.10–0.15) and 0.10 (0.07–0.14) compared with β-blocker, 0.51 (0.44–0.59) and 0.43 (0.32–0.58) compared with diuretic, and 0.97 (0.81–1.16) and 0.78 (0.56–1.08) compared with calcium-antagonist monotherapy. Risk of stroke did not differ among the five antihypertensive medications.
Use of ACEis and ARBs compared with β-blockers and diuretics associates with a reduced risk of atrial fibrillation, but not stroke, within the limitations of a retrospective study reporting associations. This suggests that controlling activation of the renin-angiotensin system in addition to controlling blood pressure is associated with a reduced risk of atrial fibrillation.
See page 1169 for the editorial comment on this article (doi:10.1093/eurheartj/ehu068)
Introduction
Hypertension is the most prevalent independent and potentially modifiable risk factor for atrial fibrillation,1 and up to 70% of patients with atrial fibrillation have a history of hypertension.2 Despite the close link between hypertension and atrial fibrillation, the underlying pathophysiology of atrial fibrillation in patients with hypertension remains unclear. In patients with hypertension, the two major mechanisms thought to lead to the development of atrial fibrillation are: (1) stretch and hemodynamic changes in the atria due to diastolic dysfunction and left atrial enlargement, and (2) activation of the renin-angiotensin system.3 All classes of antihypertensive medication may potentially reduce the risk of atrial fibrillation,4–6 but some studies have suggested that drugs targeting the renin-angiotensin system may be particularly favourable because of their effect on atrial remodelling.4,5
Six randomized hypertension trials investigating the risk of atrial fibrillation in hypertensive patients receiving renin-angiotensin system blockade [angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB)] compared with patients receiving other classes of antihypertensive medication or placebo have reported conflicting results.7–12 In the six trials, individuals with risk factors for atrial fibrillation were included and they reported either reduced risk of atrial fibrillation or no difference in risk. Exclusion of individuals with such risk factors was, however, done in an atrial fibrillation trial and in an English nationwide, nested case-control hypertension study similar to the present study.13,14 In spite of this, results were not similar, as the atrial fibrillation trial reported no difference in risk of atrial fibrillation for hypertensive patients treated with ARB or placebo and the English nationwide study showed a reduced risk of atrial fibrillation for hypertensive patients treated with ACEis, ARBs, or β-blockers compared with hypertensive patients treated with calcium-channel blockers. Thus, first, it remains unclear whether the reduced risk of atrial fibrillation associated with antihypertensive treatment is primarily due to controlling blood pressure and haemodynamic changes or due to the controlling activation of the renin-angiotensin system. Second, if renin-angiotensin system blockade is associated with the reduced risk of atrial fibrillation, it remains unclear whether the effect is present in hypertensive individuals free of other diseases predisposing to atrial fibrillation.
We examined the associations between antihypertensive treatment with ACEis or ARBs, β-blockers, diuretics, or calcium-antagonists, and risk of atrial fibrillation. For this purpose, we identified all individuals in the Danish population from 1995 through 2010 treated with only one class of antihypertensive medication, and matched individuals treated with ACEis 1:1 with individuals treated with β-blockers, diuretics, calcium-antagonists, or ARBs. Likewise, individuals treated with ARBs were matched 1:1 with individuals treated with β-blockers, diuretics, calcium-antagonists, or ACEis. We excluded the use of medication within these classes if the specific medication could also be used to treat atrial fibrillation. All individuals were free of atrial fibrillation and of predisposing diseases like heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline, and none received any other antihypertensive medication than the one examined.
Methods
Study population
We used data from the national Danish Civil Registration System, the national Danish Patient Registry, the national Danish Registry of Medicinal Products Statistics, and Statistics Denmark for the 6.7 million people living in Denmark from 1995 through 2010, as done previously.15–19 During this period, all four registries were complete, that is, for practical purposes no individuals were lost to follow-up. The study complies with the Declaration of Helsinki. Nationwide anonymous studies like the present do not need ethical approval in Denmark.
Study design
We conducted a nationwide nested 1:1 matched study among individuals with hypertension, defined as individuals receiving ACEi, ARB, β-blocker, diuretic, or calcium-antagonist monotherapy, who were not suffering from atrial fibrillation or diseases predisposing to atrial fibrillation at baseline, i.e. heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism. We did not exclude individuals with valvular heart disease. We matched individuals on ACEi monotherapy 1:1 with individuals on β-blocker, diuretic, calcium-antagonist, and ARB monotherapy based on gender, age at first dispense of antihypertensive medication (5-year intervals; if 1-year interval were used, results were similar but fewer individuals were matched), calendar-year, absence of other diseases predisposing to atrial fibrillation, and propensity score. Both individuals in each matched nested set were followed from the date of their individual first dispense of antihypertensive medication until a diagnosis of the endpoint of interest (atrial fibrillation or stroke), death (censoring), emigration (censoring), or end of follow-up on 31 December 2010 (censoring), whichever came first. The propensity-score matching was performed in an attempt to address differences in medical history between users of the five different classes of antihypertensive medication as done previously.17 For this purpose, we used pharmaceutical products mainly prescribed by the individual's family doctor, who knows the person and family medical history, dispensed before the first dispense of antihypertensive medication. Medication used in the propensity-score matching were statin (C10AA01-07; simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, cerivastatin, rosuvastatin), bronchial dilators for chronic obstructive pulmonary disease and asthma (R03AC; salbutamol, terbutaline, fenoterol, rimiterol, hexoprenaline, isoetarine, pirbuterol, tretoquinol, carbuterol, tulobuterol, salmeterol, formoterol, clenbuterol, reproterol, procaterol, bitolterol, indacaterol), and antidepressants (N06; non-selective serotonin reuptake inhibitors, selective serotonin reuptake inhibitors, non-selective monoamine oxidase inhibitors, monoamine oxidase A inhibitors, other antidepressants). These pharmaceutical products represent a wide range of illnesses often treated in the outpatient setting by the family doctor—similar to antihypertensive medication. Likewise, individuals on ARB monotherapy were matched 1:1 with individuals on β-blocker, diuretic, calcium-antagonist, and ACEi monotherapy.
Medication
We identified individuals on antihypertensive monotherapy through the national Danish Registry of Medicinal Products Statistics as individuals receiving only one class of the five different classes of antihypertensive medications. This registry records information about all prescribed drugs dispensed at all Danish pharmacies. Drugs administered during a hospital admission are not included, but immediately on discharge patients purchase their own medication in Denmark. We obtained information about the use of β-blockers [Anatomical Therapeutic Chemical Classification System (ATC): C07AA01-03, C07AA05-07, C07AA16, C07AB02-05, C07AB07, C07AB12], diuretics (ATC: C03AA01, C03AB01, C03BA11, C03CA01-02, C03CB02, C03DA01, C03DA04, C03EA01), calcium-antagonists (ATC: C08DA01, C08CA01-06, C08CA08-10, C08CA13, C08DB01), ACEis (ATC: C09AA01-07, C09AA09-10, C09AA13), and ARBs (ATC: C09CA01-04, C09CA6-08) (see Supplementary material online, Table S1, which also includes specific drug names). Individuals with dispenses of antihypertensive medications used in atrial fibrillation, i.e. verapamil (group I calcium-antagonist) and sotalol (non-selective β-blocker), were excluded from the analyses.
Diagnoses
Individuals with atrial fibrillation, heart failure, ischaemic heart disease, diabetes mellitus, hyperthyroidism, chronic kidney disease, renal hypertension, and/or stroke were identified using the national Danish Patient Registry or the national Danish Causes of Death Registry. A diagnosis of atrial fibrillation was defined as irregular and uncoordinated atrial electrical activity on a surface electrocardiogram and, in patients with intact atrioventricular conduction, the presence of an irregular ventricular response.20 A previous study including individuals from a Danish population cohort have shown that of all atrial fibrillation events, 74% were identified through the national Danish Patient Registry alone 26% through the national Danish Patient Registry and at one of the study examinations, whereas none was diagnosed solely at a study examination.21 This means that each individual diagnosed with a new-onset event of atrial fibrillation at one of the study examinations was afterwards referred to a hospital and thus registered in the national Danish Patient Registry. The national Danish Patient Registry captures all hospital visits (inpatients and outpatients) in the entire country for all persons living in Denmark. From 1995 through 2010, this registry also includes information from emergency wards. Diagnoses were classified according to the International Classification of Diseases 10th edition codes; atrial fibrillation: I48.0–I48.9; heart failure: I50.0–I50.9; ischaemic heart disease: I20–I25; diabetes mellitus: E10, E11, E13, E14; hyperthyroidism: E05.0–E05.9; chronic kidney disease: N18.0–N18.9; renal hypertension: I15.0; stroke (including stroke, transient ischaemic attacks, and amaurosis fugax): I60–I68, G45. Stroke was rapidly developed signs of focal (or global) disturbance of cerebral function lasting >24 h (unless interrupted by death), with no apparent non-vascular cause.22 A transient ischaemic attack was based on similar symptoms but lasting <24 h, while amaurosis fugax was transient blindness on one eye only.
Other covariates
The national Danish Civil Registration System records all births, immigrations, emigrations, and deaths in Denmark through the civil registration number, uniquely identifying each inhabitant in Denmark and including information on age and gender. For other characteristics, we collected data from Statistics Denmark on ethnicity (Danish ethnicity is defined as having Danish citizenship, birthplace in Denmark and with both parents also having Danish citizenship and birthplace in Denmark), highest obtained level of education, and geographical residency (size of city or rural for the longest period of residency). Through the national Danish Registry of Medicinal Products Statistics, we collected information about all prescribed drugs dispensed at all Danish pharmacies in order to calculate a mean consumption of defined daily dose in each group, and through the national Danish Patient Registry and Statistics Denmark, we collected information on congestive heart failure, hypertension, age, diabetes mellitus, stroke/transient ischaemic attacks, vascular disease, and gender in order to calculate CHA2DS2-vascular score (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, sex category).23
Statistical analyses
Data were analysed using STATA 12.1. All analyses were performed on nested 1:1 matched studies within the entire Danish population from 1995 through 2010.
First, we studied the cumulative incidence of atrial fibrillation as a function of follow-up time, allowing for competing risk of death, and used log-rank statistics for comparison. Second, we assessed incidence per year of atrial fibrillaiton as a function of calendar-year and used a nonparametric test for trend by Cuzick. Third, we used Cox regression models with delayed entry at the date of the first dispense of the antihypertensive medication to the patient to study risk of atrial fibrillation and stroke. Individuals with the relevant endpoint before study entry were excluded. We matched perfectly for age at first dispense of antihypertensive medication and gender and adjusted multivariably for defined daily dose, ethnicity, highest obtained level of education, and geographical residency; paired matching was included in the model as a stratification variable. We visually assessed the assumption of proportional hazards graphically by plotting log (cumulative incidence) for each endpoint as a function of age; no major violation of the assumption was observed. Risk of stroke influenced by lowering blood pressure rather than renin angiotensin system blockade per se was used as an indicator of the importance of blood pressure lowering per se. As atrial fibrillation in itself is a risk factor for thromboembolic stroke, individuals with atrial fibrillation were excluded from these analyses.
In sensitivity analyses, we first included sotalol in the β-blocker group and verapamil in the calcium-antagonist group. Second, as alprenolol and oxprenolol may also be used in atrial fibrillation in Denmark, we performed an analysis with sotalol, alprenolol, and oxprenolol excluded from the β-blocker group. Third, as atenolol and bisoprolol may also be used in atrial fibrillation, we performed an analysis with sotalol, atenolol, and bisoprolol excluded from the β-blocker group. Fourth, we included individuals in antihypertensive monotherapy with diseases predisposing to atrial fibrillation (heart failure, ischaemic heart disease, diabetes mellitus, or hyperthyroidism), individuals that were excluded from the main analyses. Fifth, we calculated hazard ratios of atrial fibrillation and stroke among individuals using β-blocker, calcium-antagonist, and diuretic monotherapy. Sixth, we adjusted additionally for new onset risk factors for atrial fibrillation during follow-up, for chronic kidney disease and renal hypertension at baseline and during follow-up, as well as for any hospitalization during follow-up. Seventh, we used information on atrial fibrillation treatments which are anticoagulant treatment (vitamin K antagonists, ATC: B01AA; dabigatran, ATC: B01AE07; rivaroxaban, ATC: B01AX06; acetylsalicylic acid, ATC: B01AC06; clopidogrel, ATC: B01AC04), antiarrhythmic treatment (digoxin, ATC: C01AA05; propafenone, ATC: C01BC03; flecainide, ATC: C01BC04; amiodarone, ATC: C01BD01; dronedarone, ATC: C01BD07), electrical cardioversion [Danish Health Authority Classification System (SKS): BFC, BFF], and ablation (SKS: KFPA, KFPB, KFPD) to validate the atrial fibrillation diagnosis. For this sensitivity analysis, an atrial fibrillation event was an atrial fibrillation diagnosis in the national Danish Patient Registry plus minimum one of the above mentioned treatments for atrial fibrillation. Finally, we conducted Cox regression models in strata of gender and age.
Results
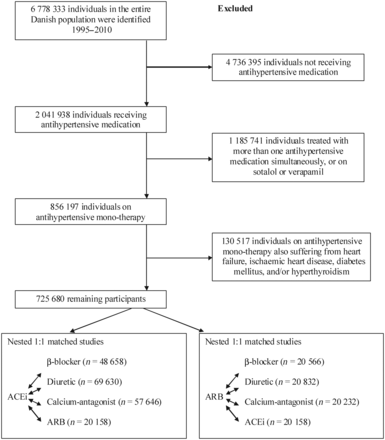
Exclusion and inclusion of study participants are shown in Figure 1. Baseline characteristics of individuals free of atrial fibrillation, heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism in the nested 1:1 matched studies within the entire Danish population 1995 through 2010 are shown in Table 1 and Supplementary material online, Table S2. Baseline characteristics of all individuals free of atrial fibrillation, heart failure, ischaemic heart disease, diabetes mellitus, and/or hyperthyroidism on antihypertensive monotherapy and of the entire Danish population are shown in Supplementary material online, Table S3.
Baseline characteristics of individuals on antihypertensive monotherapy used in nested 1:1 matched studies within the entire Danish population 1995 through 2010
| . | ACEi vs. β-blocker . | ACEi vs. diuretic . | ACEi vs. calcium-antagonist . | ACEi vs. ARB . | ||||
|---|---|---|---|---|---|---|---|---|
| Number | 24 329 | 24 329 | 34 815 | 34 815 | 28 823 | 28 823 | 10 079 | 10 079 |
| Women (%) | 46 | 46 | 46 | 46 | 43 | 43 | 42 | 42 |
| Age at first medication | 56 (46–66) | 56 (46–66) | 58 (49–67) | 58 (49–67) | 59 (50–68) | 59 (50–68) | 56 (48–65) | 56 (48–65) |
| Dose, mean DDD | 1.2 (0.6–1.6) | 0.4 (0.3–0.6) | 1.2 (0.6–1.7) | 0.9 (0.5–1.1) | 1.2 (0.6–1.7) | 1.1 (0.9–1.4) | 1.2 (0.6–1.6) | 1.0 (0.7–1.2) |
| Chronic kidney disease (%) | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.08 |
| Renal hypertension (%) | 0.004 | 0.008 | 0.003 | 0.009 | 0.004 | 0.001 | 0.01 | 0 |
| CHA2DS2-Vasc score (mean) | 2.8 (2–3) | 2.7 (2–3) | 2.8 (2–3) | 2.8 (2-3) | 2.8 (2-3) | 2.8 (2-3) | 2.7 (2-3) | 2.7 (2-3) |
| Ethnicity (%) | ||||||||
| Danish | 94 | 94 | 94 | 96 | 94 | 94 | 94 | 94 |
| Other | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 |
| Residential city size (%) | ||||||||
| <12 000 or rural | 40 | 43 | 41 | 42 | 41 | 41 | 41 | 37 |
| 12 000–100 000 | 28 | 29 | 28 | 27 | 28 | 27 | 28 | 26 |
| >100 000 | 32 | 28 | 31 | 31 | 31 | 32 | 32 | 37 |
| Level of education (%) | ||||||||
| Not available | 6 | 7 | 6 | 6 | 6 | 6 | 5 | 5 |
| Primary and high school | 35 | 36 | 35 | 42 | 35 | 35 | 35 | 30 |
| Vocational | 37 | 35 | 37 | 35 | 37 | 37 | 38 | 37 |
| Academic | 22 | 22 | 22 | 17 | 22 | 22 | 22 | 28 |
| . | ACEi vs. β-blocker . | ACEi vs. diuretic . | ACEi vs. calcium-antagonist . | ACEi vs. ARB . | ||||
|---|---|---|---|---|---|---|---|---|
| Number | 24 329 | 24 329 | 34 815 | 34 815 | 28 823 | 28 823 | 10 079 | 10 079 |
| Women (%) | 46 | 46 | 46 | 46 | 43 | 43 | 42 | 42 |
| Age at first medication | 56 (46–66) | 56 (46–66) | 58 (49–67) | 58 (49–67) | 59 (50–68) | 59 (50–68) | 56 (48–65) | 56 (48–65) |
| Dose, mean DDD | 1.2 (0.6–1.6) | 0.4 (0.3–0.6) | 1.2 (0.6–1.7) | 0.9 (0.5–1.1) | 1.2 (0.6–1.7) | 1.1 (0.9–1.4) | 1.2 (0.6–1.6) | 1.0 (0.7–1.2) |
| Chronic kidney disease (%) | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.08 |
| Renal hypertension (%) | 0.004 | 0.008 | 0.003 | 0.009 | 0.004 | 0.001 | 0.01 | 0 |
| CHA2DS2-Vasc score (mean) | 2.8 (2–3) | 2.7 (2–3) | 2.8 (2–3) | 2.8 (2-3) | 2.8 (2-3) | 2.8 (2-3) | 2.7 (2-3) | 2.7 (2-3) |
| Ethnicity (%) | ||||||||
| Danish | 94 | 94 | 94 | 96 | 94 | 94 | 94 | 94 |
| Other | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 |
| Residential city size (%) | ||||||||
| <12 000 or rural | 40 | 43 | 41 | 42 | 41 | 41 | 41 | 37 |
| 12 000–100 000 | 28 | 29 | 28 | 27 | 28 | 27 | 28 | 26 |
| >100 000 | 32 | 28 | 31 | 31 | 31 | 32 | 32 | 37 |
| Level of education (%) | ||||||||
| Not available | 6 | 7 | 6 | 6 | 6 | 6 | 5 | 5 |
| Primary and high school | 35 | 36 | 35 | 42 | 35 | 35 | 35 | 30 |
| Vocational | 37 | 35 | 37 | 35 | 37 | 37 | 38 | 37 |
| Academic | 22 | 22 | 22 | 17 | 22 | 22 | 22 | 28 |
DDD, defined daily dose; CHA2DS2-Vasc score, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, sex category. Danish ethnicity is defined as having Danish citizenship, birthplace in Denmark and with both parents also having Danish citizenship and birthplace in Denmark. Residential city size designates the location for longest period of residency. Level of education is the highest obtained level. Individuals with atrial fibrillation, heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline were excluded. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Baseline characteristics of individuals on antihypertensive monotherapy used in nested 1:1 matched studies within the entire Danish population 1995 through 2010
| . | ACEi vs. β-blocker . | ACEi vs. diuretic . | ACEi vs. calcium-antagonist . | ACEi vs. ARB . | ||||
|---|---|---|---|---|---|---|---|---|
| Number | 24 329 | 24 329 | 34 815 | 34 815 | 28 823 | 28 823 | 10 079 | 10 079 |
| Women (%) | 46 | 46 | 46 | 46 | 43 | 43 | 42 | 42 |
| Age at first medication | 56 (46–66) | 56 (46–66) | 58 (49–67) | 58 (49–67) | 59 (50–68) | 59 (50–68) | 56 (48–65) | 56 (48–65) |
| Dose, mean DDD | 1.2 (0.6–1.6) | 0.4 (0.3–0.6) | 1.2 (0.6–1.7) | 0.9 (0.5–1.1) | 1.2 (0.6–1.7) | 1.1 (0.9–1.4) | 1.2 (0.6–1.6) | 1.0 (0.7–1.2) |
| Chronic kidney disease (%) | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.08 |
| Renal hypertension (%) | 0.004 | 0.008 | 0.003 | 0.009 | 0.004 | 0.001 | 0.01 | 0 |
| CHA2DS2-Vasc score (mean) | 2.8 (2–3) | 2.7 (2–3) | 2.8 (2–3) | 2.8 (2-3) | 2.8 (2-3) | 2.8 (2-3) | 2.7 (2-3) | 2.7 (2-3) |
| Ethnicity (%) | ||||||||
| Danish | 94 | 94 | 94 | 96 | 94 | 94 | 94 | 94 |
| Other | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 |
| Residential city size (%) | ||||||||
| <12 000 or rural | 40 | 43 | 41 | 42 | 41 | 41 | 41 | 37 |
| 12 000–100 000 | 28 | 29 | 28 | 27 | 28 | 27 | 28 | 26 |
| >100 000 | 32 | 28 | 31 | 31 | 31 | 32 | 32 | 37 |
| Level of education (%) | ||||||||
| Not available | 6 | 7 | 6 | 6 | 6 | 6 | 5 | 5 |
| Primary and high school | 35 | 36 | 35 | 42 | 35 | 35 | 35 | 30 |
| Vocational | 37 | 35 | 37 | 35 | 37 | 37 | 38 | 37 |
| Academic | 22 | 22 | 22 | 17 | 22 | 22 | 22 | 28 |
| . | ACEi vs. β-blocker . | ACEi vs. diuretic . | ACEi vs. calcium-antagonist . | ACEi vs. ARB . | ||||
|---|---|---|---|---|---|---|---|---|
| Number | 24 329 | 24 329 | 34 815 | 34 815 | 28 823 | 28 823 | 10 079 | 10 079 |
| Women (%) | 46 | 46 | 46 | 46 | 43 | 43 | 42 | 42 |
| Age at first medication | 56 (46–66) | 56 (46–66) | 58 (49–67) | 58 (49–67) | 59 (50–68) | 59 (50–68) | 56 (48–65) | 56 (48–65) |
| Dose, mean DDD | 1.2 (0.6–1.6) | 0.4 (0.3–0.6) | 1.2 (0.6–1.7) | 0.9 (0.5–1.1) | 1.2 (0.6–1.7) | 1.1 (0.9–1.4) | 1.2 (0.6–1.6) | 1.0 (0.7–1.2) |
| Chronic kidney disease (%) | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.08 |
| Renal hypertension (%) | 0.004 | 0.008 | 0.003 | 0.009 | 0.004 | 0.001 | 0.01 | 0 |
| CHA2DS2-Vasc score (mean) | 2.8 (2–3) | 2.7 (2–3) | 2.8 (2–3) | 2.8 (2-3) | 2.8 (2-3) | 2.8 (2-3) | 2.7 (2-3) | 2.7 (2-3) |
| Ethnicity (%) | ||||||||
| Danish | 94 | 94 | 94 | 96 | 94 | 94 | 94 | 94 |
| Other | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 |
| Residential city size (%) | ||||||||
| <12 000 or rural | 40 | 43 | 41 | 42 | 41 | 41 | 41 | 37 |
| 12 000–100 000 | 28 | 29 | 28 | 27 | 28 | 27 | 28 | 26 |
| >100 000 | 32 | 28 | 31 | 31 | 31 | 32 | 32 | 37 |
| Level of education (%) | ||||||||
| Not available | 6 | 7 | 6 | 6 | 6 | 6 | 5 | 5 |
| Primary and high school | 35 | 36 | 35 | 42 | 35 | 35 | 35 | 30 |
| Vocational | 37 | 35 | 37 | 35 | 37 | 37 | 38 | 37 |
| Academic | 22 | 22 | 22 | 17 | 22 | 22 | 22 | 28 |
DDD, defined daily dose; CHA2DS2-Vasc score, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, sex category. Danish ethnicity is defined as having Danish citizenship, birthplace in Denmark and with both parents also having Danish citizenship and birthplace in Denmark. Residential city size designates the location for longest period of residency. Level of education is the highest obtained level. Individuals with atrial fibrillation, heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline were excluded. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Exclusion and inclusion of study participants from the entire Danish population from 1995 through 2010. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
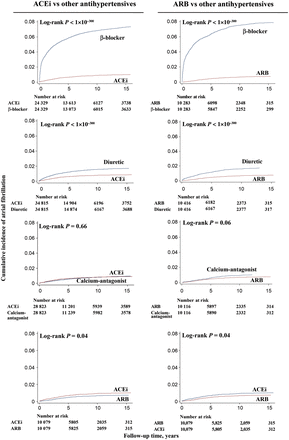
Cumulative incidence of atrial fibrillation
ACEi and ARB monotherapy were both associated with lower cumulative incidences of atrial fibrillation compared with β-blocker and diuretic monotherapy, but not compared with calcium-antagonists (Figure 2). Median follow-up times for ACEi and ARB monotherapy were 6.8 years (interquartile range 3.0–9.7) and 6.6 years (5.9–9.2) when compared with β-blocker, 5.9 years (2.0–8.8) and 6.7 years (3.4–9.4) when compared with diuretic, and 5.9 years (2.4–7.9) and 6.6 years (3.3–9.5) when compared with calcium-antagonist monotherapy. Median follow-up time for ACEi compared with ARB monotherapy was 6.5 years (3.3–8.9). Incidence rates for the individual classes of antihypertensive medication in the individual nested 1:1 matched studies are shown in Table 2.
Incidence rate of atrial fibrillation and stroke per 10 000 person-years
| . | Incidence rate of atrial fibrillation per 10 000 person-years . | Incidence rate of stroke per 10 000 person-years . |
|---|---|---|
| ACEi vs. β-blocker | 15.1 (13.5–16.9) 99.1 (94.8–103.5) | 72.8 (69.0–76.7) 63.8 (60.3–67.4) |
| ACEi vs. Diuretic | 16.0 (14.4–17.7) 31.4 (29.2–33.8) | 74.0 (70.4–77.8) 71.5 (67.9–75.2) |
| ACEi vs. calcium-antagonist | 16.4 (14.6–18.3) 17.8 (16.0–19.9) | 76.6 (72.5–80.9) 72.8 (68.8–77.0) |
| ACEi vs. ARB | 15.4 (12.8–18.5) 11.9 (9.6–14.7) | 62.0 (56.3–68.3) 60.3 (54.7–66.5) |
| ARB vs. β-blocker | 11.8 (9.6–14.5) 118.5 (110.8–126.7) | 59.6 (54.1–65.5) 59.1 (53.6–65.2) |
| ARB vs. diuretic | 12.1 (9.9–14.5) 27.1 (23.6–31.0) | 60.6 (55.1–66.6) 67.5 (61.6–73.8) |
| ARB vs. calcium-antagonist | 12.2 (9.9–15.0) 16.2 (13.5–19.4) | 61.5 (55.9–67.7) 66.3 (60.3–72.8) |
| ARB vs. ACEi | 11.7 (9.6–14.7) 15.4 (12.8–18.5) | 60.3 (54.7–66.5) 62.0 (56.3–68.3) |
| . | Incidence rate of atrial fibrillation per 10 000 person-years . | Incidence rate of stroke per 10 000 person-years . |
|---|---|---|
| ACEi vs. β-blocker | 15.1 (13.5–16.9) 99.1 (94.8–103.5) | 72.8 (69.0–76.7) 63.8 (60.3–67.4) |
| ACEi vs. Diuretic | 16.0 (14.4–17.7) 31.4 (29.2–33.8) | 74.0 (70.4–77.8) 71.5 (67.9–75.2) |
| ACEi vs. calcium-antagonist | 16.4 (14.6–18.3) 17.8 (16.0–19.9) | 76.6 (72.5–80.9) 72.8 (68.8–77.0) |
| ACEi vs. ARB | 15.4 (12.8–18.5) 11.9 (9.6–14.7) | 62.0 (56.3–68.3) 60.3 (54.7–66.5) |
| ARB vs. β-blocker | 11.8 (9.6–14.5) 118.5 (110.8–126.7) | 59.6 (54.1–65.5) 59.1 (53.6–65.2) |
| ARB vs. diuretic | 12.1 (9.9–14.5) 27.1 (23.6–31.0) | 60.6 (55.1–66.6) 67.5 (61.6–73.8) |
| ARB vs. calcium-antagonist | 12.2 (9.9–15.0) 16.2 (13.5–19.4) | 61.5 (55.9–67.7) 66.3 (60.3–72.8) |
| ARB vs. ACEi | 11.7 (9.6–14.7) 15.4 (12.8–18.5) | 60.3 (54.7–66.5) 62.0 (56.3–68.3) |
Incidence rate of atrial fibrillation and stroke per 10 000 person-years
| . | Incidence rate of atrial fibrillation per 10 000 person-years . | Incidence rate of stroke per 10 000 person-years . |
|---|---|---|
| ACEi vs. β-blocker | 15.1 (13.5–16.9) 99.1 (94.8–103.5) | 72.8 (69.0–76.7) 63.8 (60.3–67.4) |
| ACEi vs. Diuretic | 16.0 (14.4–17.7) 31.4 (29.2–33.8) | 74.0 (70.4–77.8) 71.5 (67.9–75.2) |
| ACEi vs. calcium-antagonist | 16.4 (14.6–18.3) 17.8 (16.0–19.9) | 76.6 (72.5–80.9) 72.8 (68.8–77.0) |
| ACEi vs. ARB | 15.4 (12.8–18.5) 11.9 (9.6–14.7) | 62.0 (56.3–68.3) 60.3 (54.7–66.5) |
| ARB vs. β-blocker | 11.8 (9.6–14.5) 118.5 (110.8–126.7) | 59.6 (54.1–65.5) 59.1 (53.6–65.2) |
| ARB vs. diuretic | 12.1 (9.9–14.5) 27.1 (23.6–31.0) | 60.6 (55.1–66.6) 67.5 (61.6–73.8) |
| ARB vs. calcium-antagonist | 12.2 (9.9–15.0) 16.2 (13.5–19.4) | 61.5 (55.9–67.7) 66.3 (60.3–72.8) |
| ARB vs. ACEi | 11.7 (9.6–14.7) 15.4 (12.8–18.5) | 60.3 (54.7–66.5) 62.0 (56.3–68.3) |
| . | Incidence rate of atrial fibrillation per 10 000 person-years . | Incidence rate of stroke per 10 000 person-years . |
|---|---|---|
| ACEi vs. β-blocker | 15.1 (13.5–16.9) 99.1 (94.8–103.5) | 72.8 (69.0–76.7) 63.8 (60.3–67.4) |
| ACEi vs. Diuretic | 16.0 (14.4–17.7) 31.4 (29.2–33.8) | 74.0 (70.4–77.8) 71.5 (67.9–75.2) |
| ACEi vs. calcium-antagonist | 16.4 (14.6–18.3) 17.8 (16.0–19.9) | 76.6 (72.5–80.9) 72.8 (68.8–77.0) |
| ACEi vs. ARB | 15.4 (12.8–18.5) 11.9 (9.6–14.7) | 62.0 (56.3–68.3) 60.3 (54.7–66.5) |
| ARB vs. β-blocker | 11.8 (9.6–14.5) 118.5 (110.8–126.7) | 59.6 (54.1–65.5) 59.1 (53.6–65.2) |
| ARB vs. diuretic | 12.1 (9.9–14.5) 27.1 (23.6–31.0) | 60.6 (55.1–66.6) 67.5 (61.6–73.8) |
| ARB vs. calcium-antagonist | 12.2 (9.9–15.0) 16.2 (13.5–19.4) | 61.5 (55.9–67.7) 66.3 (60.3–72.8) |
| ARB vs. ACEi | 11.7 (9.6–14.7) 15.4 (12.8–18.5) | 60.3 (54.7–66.5) 62.0 (56.3–68.3) |
Cumulative incidences of atrial fibrillation as a function of follow-up time in nested 1:1 matched studies within the entire Danish population from 1995 through 2010. Calculations of cumulative incidence allowed for competing risk of death. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
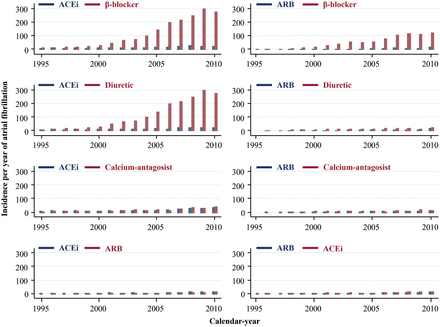
Incidence rates of atrial fibrillation as a function of calendar-year
Incidence rates of atrial fibrillation as a function of calendar-year increased more in the groups treated with β-blocker or diuretic monotherapy compared with incidence rates of atrial fibrillation in the other three groups of antihypertensive monotherapy (Figure 3).
Incidence per year of atrial fibrillation in nested 1:1 matched studies within the entire Danish population as a function of calendar-year (1995 through 2010). ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
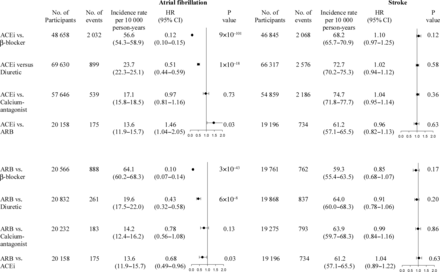
Risk of atrial fibrillation and stroke
The hazard ratio of atrial fibrillation for ACEi monotherapy was 0.12 (95% CI: 0.10–0.15) compared with β-blocker, 0.51 (0.44–0.59) compared with diuretic, 0.97 (0.81–1.16) compared with calcium-antagonist, and 1.46 (1.04–2.05) compared with ARB monotherapy (Figure 4). In contrast, for risk of stroke as an endpoint influenced by lowering blood pressure rather than renin-angiotensin system blockade per se, hazard ratios for ACEi monotherapy vs. other antihypertensive monotherapies did not differ from 1.0 (Figure 4).
Risk of atrial fibrillation and stroke in nested 1:1 matched studies within the entire Danish population from 1995 through 2010. Individuals with atrial fibrillation were excluded from the analyses of stroke risk. Hazard ratios were adjusted multivariably for defined daily dose, ethnicity, highest obtained level of education, geographical residency, and paired matching was included in the model as a stratification variable, in the studies matched for antihypertensive medication, gender, age at first prescription, and propensity score. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; HR, hazard ratio; CI, confidence interval.
The hazard ratio of atrial fibrillation for ARB monotherapy was 0.10 (95% CI: 0.07–0.14) compared with β-blocker, 0.43 (0.32–0.58) compared with diuretic, 0.78 (0.56–1.08) compared with calcium-antagonist, and 0.68 (0.49–0.96) compared with ACEi monotherapy (Figure 4). In contrast, for risk of stroke, hazard ratios for ARB monotherapy vs. other antihypertensive monotherapies did not differ from 1.0 (Figure 4). Corresponding comparisons among β-blocker, calcium-antagonist, and diuretic monotherapy are presented in Supplementary material online, Figure S5.
Sensitivity analyses
First, when β-blocker monotherapy included sotalol (see Supplementary material online, Figure S1), results were similar to those shown in Figure 4; however, when calcium-antagonists included verapamil hazard ratios of atrial fibrillation associated with ACEi and ARB monotherapy were 0.52 (0.44–0.60) and 0.41 (0.30–0.54) (see Supplementary material online, Figure S1), whereas corresponding hazard ratios were 0.97 (0.81–1.16) and 0.78 (0.56–1.08) when verapamil was excluded (Figure 4). Second, when sotalol, alprenolol, and oxprenolol were excluded from the β-blocker group (see Supplementary material online, Figure S2), results were similar to Figure 4. Third, when sotalol, atenolol, and bisoprolol were excluded from the β-blocker group (see Supplementary material online, Figure S3), results were similar to Figure 4. Fourth, when individuals with diseases predisposing to atrial fibrillation were included (see Supplementary material online, Figure S4), results were similar to those shown in Figure 4. The only exception was a slight increase in risk of stroke associated with ACEi monotherapy compared with β-blocker monotherapy (see Supplementary material online, Figure S4). Fifth, when we calculated hazard ratios of atrial fibrillation and stroke among β-blocker, calcium-antagonist, and diuretic monotherapy β-blocker monotherapy was associated with the increased risk of atrial fibrillation compared with calcium-antagonists and diuretics (see Supplementary material online, Figure S5). Sixth, when adjustment additionally included new onset risk factors for atrial fibrillation during follow-up and hospitalizations due to any cause during follow-up (see Supplementary material online, Tables S4 and S5) and for chronic kidney disease and renal hypertension at baseline (Table 1 and Supplementary material online, Table S2), results were similar (compare Supplementary material online, Figure S6 with Figure 4). Seventh, when limiting the analysis to only validated atrial fibrillation diagnoses (see Supplementary material online, Figure S7), results were similar to those shown in Figure 4. Finally, hazard ratios of atrial fibrillation were similar in strata of age and gender compared with overall analyses (see Supplementary material online, Figure S8).
Discussion
The main finding of this study is that ACEis and ARBs, when used as monotherapy in hypertensive patients without heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline, possibly can contribute to the prevention of atrial fibrillation in the long term compared with monotherapy with a β-blocker. Importantly, this finding is within the limitations of a retrospective study reporting associations; that is, β-blockers may have been selected based on a suspicion that atrial fibrillation could develop and thus have been preferred for hypertensive individuals that report palpitations. Incidence rates of atrial fibrillation as a function of calendar-year increased more in the groups treated with β-blocker or diuretic monotherapy compared with incidence rates of atrial fibrillation in all the other groups of antihypertensive monotherapy, suggesting that these antihypertensives may indeed have been selected for individuals with yet undiagnosed atrial fibrillation. Thus, importantly, as this study is retrospective in nature and prone to confounders, these results should be interpreted cautiously.
Hypertension is, besides being a major risk factor for atrial fibrillation, also the single most important risk factor for stroke.24 Using stroke as a ‘control’ for the effect of lowering blood pressure rather than renin-angiotensin system blockade per se revealed that none of the five antihypertensive medications compared with the others associated with reduced stroke risk. These findings thus suggest that controlling activation of the renin-angiotensin system in addition to controlling blood pressure and thereby haemodynamic changes may help reduce the risk of atrial fibrillation.
A novel aspect of this study is that we compared both ACEis and ARBs with all of the other four classes of antihypertensive medications, in large groups of individuals on antihypertensive monotherapy encompassing the entire Danish population from 1995 through 2010. In contrast, in randomized clinical trials, normally only two of the five established classes of antihypertensive medication are compared, and then only in individuals willing to and found eligible to participate in the trial. The Danish registries provided important information on potential confounders such as age at first dispense of antihypertensive medication, defined daily dose, gender, ethnicity, highest obtained level of education, and geographical residency. This was combined into a propensity score included in the adjustment of our risk estimates.
Previous studies
Two previous hypertension trials reported reduced risk of atrial fibrillation in the group treated with renin-angiotensin system blockade,10,11 while four hypertension trials reported no difference in risk of atrial fibrillation between renin-angiotensin system blockade vs. other antihypertensive treatments or placebo.7–9,12 However, in these six hypertension trials, individuals with risk factors for atrial fibrillation were included, whereas individuals with risk factors for atrial fibrillation were excluded in our study. Exclusion of individuals with such risk factors was, however, done in an atrial fibrillation trial and in an English nationwide, nested case-control hypertension study similar to the present study.13,14 In spite of this, results were not similar, as the atrial fibrillation trial reported no difference in risk of atrial fibrillation for hypertensive patients treated with ARB or placebo and the English nationwide study showed reduced risk of atrial fibrillation for hypertensive patients treated with ACEis, ARBs, or β-blockers compared with hypertensive patients treated with calcium-channel blockers. Our study demonstrated reduced risk of atrial fibrillation for individuals treated with renin-angiotensin system blockade compared with individuals treated with β-blockers and diuretics. An explanation for this discrepancy may be found in the fact that treatment with a diuretic in addition to treatment with an ACEi, an ARB, a β-blockers, or a calcium-antagonist was not an exclusion criteria in the English nationwide study and this increases the risk of miscalculation due to confounding by the effect of other classes of antihypertensive medication taken concurrently. In our study, we circumvented this potential risk of miscalculation by including individuals on monotherapy only and our results support the 2007 ESH/ESC guidelines for the management of arterial hypertension,25 suggesting ACEis and ARBs as preferred drugs in patients with hypertension and at risk of developing atrial fibrillation. Secondary analyses of trials testing ACEis or ARBs in patients with structural heart disease including left ventricular hypertrophy convincingly show a preventive effect of ACEis and ARBs for new-onset atrial fibrillation26–28 while two randomized trials failed to show such an effect in patients with lone atrial fibrillation.29,30 Hence, the controversy is limited to whether a population of hypertensive patients without other diseases predisposing to atrial fibrillation at baseline likewise may benefit from renin-angiotensin system blockade in preventing atrial fibrillation, the question addressed in the present study.
Strengths
Strengths of the present study include the size of the initial study population of 6.7 million individuals, which provided the opportunity to only include individuals on antihypertensive monotherapy representing a low risk group with regard to atrial fibrillation of 13% of patients receiving any antihypertensive treatment and to exclude individuals suffering from atrial fibrillation, heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline. This eliminates the risk of our results being confounded by the effect of other classes of antihypertensive medication taken concurrently and enables us to study a group of individuals for whom the effect of renin-angiotensin system blockade in the prevention of atrial fibrillation is not thoroughly tested previously. Also, as we studied all available individuals in an entire population, selection bias for entry into the study is not an issue. Finally, using stroke as a ‘control’ adds credibility to our findings.
Limitations
A theoretical limitation of the study concerns the availability and completeness of the information from the national Danish registries; however, the national Danish Patient Registry captures 100% of diseases diagnosed at all hospitals in Denmark, the national Danish Registry of Medicinal Products Statistics records 100% of all dispensed prescriptions of antihypertensive medication in all pharmacies in Denmark, and the national Danish Civil Registration System captures 100% of all births, deaths, emigrations, and immigrations in Denmark. Nevertheless, since we chose to include individuals with hypertension defined as individuals receiving ACEi, ARB, β-blocker, diuretic, or calcium-antagonist monotherapy, we cannot exclude that some individuals received antihypertensive medication for other reasons than hypertension; a limitation is thus that we do not have information on why a specific treatment was selected, which may have confounded our results. Another limitation is that we did not have information on actual blood pressure control or whether the administered dose resulted in the target blood pressure and it is possible that ACEis and ARBs controlled blood pressure more effectively than β-blockers and diuretics; however, we did include adjustment for defined daily dose for each individual treated with any antihypertensive medication and to further compensate we included the endpoint stroke mainly influenced by blood pressure control, and stroke risk did not differ among the different classes of antihypertensive medication. Alternatively, the need for additional therapy with a second antihypertensive agent may provide a proxy for not reaching target blood pressure; however, we a priori excluded all participants receiving more than one antihypertensive agent, as combination therapy would make interpretation of results difficult. Also, we did not have information on patient characteristics, clinical course, parameters like albumin/creatinin ratio, left ventricular hypertrophy, or heart rate. However, we calculated a CHA2DS2-Vascular score and number of hospital admissions due to atrial fibrillation within the first year after atrial fibrillation onset and number of hospital admissions due to any cause during follow-up after initiation of antihypertensive treatment in order to provide information about co-morbidities and found no major differences among groups except for an increased prevalence of hospital admissions due to atrial fibrillation within the first year after atrial fibrillation onset in the β-blocker monotherapy group compared with both ACEi and ARB monotherapy groups. It is difficult completely to exclude that the association of β-blockers with the high prevalence of atrial fibrillation observed in the present study is not due to the application of β-blockers for rate limitation, when so far not reported atrial fibrillation occurs, or by a higher cardiovascular disease load in these individuals. Thus, an explanation for why β-blockers appear to increase the risk of atrial fibrillation in hypertensive individuals could be that β-blockers may have been selected based on a suspicion that atrial fibrillation might develop and thus have been preferred for hypertensive individuals that report palpitations, among whom some might have had undiagnosed episodes of atrial fibrillation. However, the fact that the reduced risk of atrial fibrillation associated with ACEi and ARB monotherapy compared with β-blocker monotherapy remained, even though we excluded different β-blockers potentially used in atrial fibrillation from the analyses, indicates that confounding by indication is unlikely to explain our findings. On the other hand, excluding verapamil from the main analysis seems to be a good choice, since sensitivity analysis of atrial fibrillation risk associated with ACEi monotherapy was much lower when compared with calcium-antagonist monotherapy including verapamil. The increased risk of atrial fibrillation associated with the use of diuretics could be due to symptomatic prescriptions of diuretics to individuals with yet undiagnosed heart failure, since it is a progressive disease, or due to the fact that diuretics may decrease potassium and magnesium blood levels with consequent resulting increased risk of atrial fibrillation. Thus, clearly we cannot completely exclude confounding by indication in all our findings. However, we even matched individuals on a propensity score aimed at addressing differences in medical history between users of the five different classes of antihypertensive medication, further reducing the likelihood of bias by indication. In addition, we did not take medication interruption into consideration. The diagnosis of atrial fibrillation was obtained from registries and we were not able to distinguish between paroxysmal and permanent atrial fibrillation. We did not capture silent atrial fibrillation or atrial fibrillation diagnosed at the general practitioner, but only atrial fibrillation events leading to hospital visits either as inpatient, outpatient, or through emergency visits. Furthermore, we did not have information on the diagnostic tests applied to establish the diagnosis. Finally, as the majority of the participants were whites of Danish decent, our results may not necessarily apply to other races. On the other hand, we are not aware of data, suggesting that results like the present should not apply to all humans.
Conclusion
The main finding of this study is that ACEis and ARBs, when used as monotherapy in hypertensive patients without heart failure, ischaemic heart disease, diabetes mellitus, and hyperthyroidism at baseline, possibly can contribute to the prevention of atrial fibrillation in the long term when compared with monotherapy with a β-blocker. Importantly, this finding is within the limitations of a retrospective study reporting associations; that is, β-blockers may have been selected based on a suspicion that atrial fibrillation could develop and thus have been preferred for hypertensive individuals that report palpitations. In contrast, none of the five antihypertensive medications differed with respect to risk of stroke. These findings thus suggest that controlling activation of the renin-angiotensin system in addition to controlling blood pressure is associated with the reduced risk of atrial fibrillation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Herlev Hospital, Copenhagen University Hospital, University of Copenhagen, and The Danish Agency for Science, Technology and Innovation, The Danish Medical Research Council.
Conflict of interest: none declared.




Comments
We thank Franz H. Messerli et al. for their interest in our national study on different antihypertensive treatment strategies and the probability of developing atrial fibrillation. Comments on the study are addressed below:
1. One can undoubtedly argue that we cannot comment on the activity of the renin angiotensin system (RAS) for individuals on antihypertensive mono- therapy with either an angiotensin converting enzyme inhibitor (ACEi), an angiotensin receptor blocker (ARB), a beta-blocker, a calcium channel blockers, or a diuretic since we have not directly measured the activity of the RAS(1). However, the key-action of ACEis and ARBs is to block the RAS and we therefore consider it appropriate to believe that patients treated with either ACEi mono-therapy or ARB mono-therapy will experience a more potent RAS-inhibition compared to patients treated with beta- blocker, calcium-antagonist, or diuretic mono-therapy even though some of these medications also have the ability to influence the RAS in addition to acting through other mechanisms. Thus, we agree that our conclusion is an extrapolation. As we do not have direct measurements of the activity of the RAS, we only suggest inhibition of the RAS as a potential mechanistic explanation for the observed reduced risk of atrial fibrillation associated with ACEi and ARB mono-therapy compared with beta-blocker and diuretic mono-therapy(1).
2. We recognize that beta-blockers also have the ability to influence the RAS in addition to their predominant beta-receptor blocking effect. However, beta-blockers inhibit the RAS through another mechanism than ACEis and ARBs. Beta-blockers inhibit renin release from the kidneys and were the original inhibitors of the renin-angiotensin system, whereas ACEis and ARBs reduce angiotensin II receptor type 1 stimulation interrupting the negative feedback-mediated regulation of renin release(2), and are the most established medications for inhibition of the RAS today. We therefore chose to regard ACEis and ARBs as considerably more potent inhibitors of the RAS than beta-blockers.
3. Based on the paper by Cappuccio FP et al. enrolling six patients(3) we recognize that calcium channel blockers may stimulate the renin angiotensin system to an unknown degree. However, as the authors state, one explanation for the increase in plasma renin activity is a small reduction in total body sodium since the patients were placed on a restricted sodium intake of 150 mmol/day. A review paper from 2012 about the mechanisms by which calcium channel blockers exert pleiotropic effects, both alone and in combination with statins and inhibitors of the renin-angiotensin system, stimulation of the RAS is not mentioned(4). Thus, stimulation of the RAS by calcium-antagonists does not appear to be a key action of calcium channel blockers which could explain the surprising absence of differences in risk of atrial fibrillation between ACEi mono-therapy and ARB mono-therapy compared with calcium channel blockers mono-therapy, respectively(1).
4. We consider it appropriate to regard ACEs and ARBs as the most potent inhibitory agents of the RAS among beta-blockers, calcium- antagonists, diuretics, ACEis, and ARBs and therefore maintain our conclusion that ACEis and ARBs compared with beta-blockers and diuretics are associated with a reduced risk of atrial fibrillation, but not stroke. Our conclusion furthermore includes the suggestion that controlling activation of the renin-angiotensin system in addition to controlling blood pressure is associated with reduced risk of atrial fibrillation.
Kind regards, Sarah Marott, Sune F. Nielsen, Marianne Benn, and Boerge G. Nordestgaard.
References
1. Marott SC, Nielsen SF, Benn M, Nordestgaard BG. Antihypertensive treatment and risk of atrial fibrillation: a nationwide study. Eur Heart J 2013 Dec 17. [Epub ahead of print]
2. Campbell DJ. Renin inhibition - mechanisms of action. Experimental and clinical pharmacology 2009;32:132-135.
3. Cappuccio FP, Markandu ND, Sagnella GA, Singer DR, Buckley MG, Miller MA, Macgregor GA. Effects of amlodipine on urinary sodium excretion, renin-angiotensin-aldosterone system, atrial natriuretic peptide and blood pressure in essential hypertension. J.Hum.Hypertens. 1991;5:115-119.
4. Mason RP. Pleiotropic effects of calcium channel blockers. Cur. Hypertens. Rep 2012;14:293-303.
Conflict of Interest:
None declared
We read with interest the paper of Marott et al (1) looking at different antihypertensive treatment strategies and the probability of developing atrial fibrillation. Based on their findings that both ACE inhibitors and ARBs reduce this risk to the same extent the authors come to the conclusion that controlling activation of the renin angiotensin system in addition to controlling blood pressure was associated with the reduced risk of atrial fibrillation. We take issue with this conclusion which, as the activity of the renin angiotensin system (RAS) was not assessed in this study, must be considered an extrapolation. The authors seem to have overlooked that beta-blockers are well known to reduce the activity of the renin angiotensin system.(2-4) Yet, the largest difference in atrial fibrillation risk was seen between beta-blocker and the other RAS blockers, i.e.ARBs and ACE inhibitors. Even more importantly, calcium channel blockers (CCBs) have been documented, if anything, to stimulate the renin angiotensin system (5) and the sympathetic nervous system.(6) Yet, the authors did not find any difference in atrial fibrillation risk between RAS blockers and CCBs.(1) As the authors rightly state, one can quibble with the findings of a retrospective study reporting associations. However, if there is any conclusion to be drawn from these data, it clearly is not that controlling activation of the renin angiotensin system reduced risk of atrial fibrillation. In fact, the data by Marott et al.(1) suggest exactly the opposite, namely that controlling activation of the renin angiotensin system has no effect of the risk of atrial fibrillation.
1. Marott SC, Nielsen SF, Benn M, Nordestgaard BG. Antihypertensive treatment and risk of atrial fibrillation: a nationwide study. Eur Heart J. 2013 Dec 17. [Epub ahead of print]
2. Stokes GS, Weber MA, Thornell IR. Beta-blockers and plasma renin activity in hypertension. Br Med J. 1974 Jan 12;1(5897):60-
3. Hansson L, Zweifler AJ, Julius S, Hunyor SN. Hemodynamic effects of acute and prolonged beta-adrenergic blockade in essential hypertension. Acta Med Scand. 1974 Jul-Aug;196(1-2):27-34.
4. Kolloch R, Stumpe KO, Vetter H, Gramann W, Kruck F. Differential effects of acute and chronic beta-adrenoreceptor blockade on blood pressure and the renin-angiotensin-aldosterone system in essential hypertension. Clin Sci Mol Med Suppl 1976;3:537s-540s.
5. Cappuccio FP, Markandu ND, Sagnella GA, Singer DR, Buckley MG, Miller MA, MacGregor GA. Effects of amlodipine on urinary sodium excretion, renin -angiotensin-aldosterone system, atrial natriuretic peptide and blood pressure in essential hypertension. J Hum Hypertens. 1991 Apr;5(2):115-9.
6. Dreslinski GR, Messerli FH, Dunn FG, Suarez DH, Reisin E, Frohlich ED. Hemodynamics, biochemical and reflexive changes produced by atenolol in hypertension. Circulation. 1982 Jun;65(7):1365-8.
Conflict of Interest:
None declared