-
PDF
- Split View
-
Views
-
Cite
Cite
Lionel H. Opie, Gary D. Lopaschuk, What is good for the circulation also lessens cancer risk, European Heart Journal, Volume 36, Issue 19, 14 May 2015, Pages 1157–1162, https://doi.org/10.1093/eurheartj/ehu457
Close - Share Icon Share
Hypothesis: what is good for the circulation also lessens cancer risk
Although there have been substantial advances in the prevention and management of cardiovascular disease (CVD) and its complications, a new player and concept has entered the scene, namely an association between CVD and cancer. There were two alerting signals to this remarkable coupling. On the one hand, disconcerting evidence has been provided that suggests that the use of angiotensin receptor blockers could be increasing the development of cancers.1–3 On the other hand, evidence has been provided that the preventative effects of aspirin both as an antithrombotic agent in CVD prevention and the subsequent discovery of its effects in lessening the development of cancer, especially but not only primary gastrointestinal and distant metastases.4 In addition, ideal cardiovascular health has been shown to be inversely associated with incident cancer in the Atherosclerosis Risk In Communities (ARIC) study.5 The American Heart Association (AHA) has now widened its health goals to adherence to seven ideal heart health metrics that are aimed at lessening the incidence of both CVD and cancer as part of its 2020 goals.6 To achieve this goal, the AHA is therefore pursuing partnerships with cancer advocacy groups to achieve reductions in chronic disease prevalence. The ideal health factors are four ideal self-help health metrics and three ideal measured health metrics (an untreated total cholesterol <200 mg/dL, untreated blood pressure <120 mmHg systolic and 80 mm Hg diastolic, and untreated fasting serum glucose <100 mg/dL) (Table 1). Ambitious plans to reduce both CVD and cancer will be communicated to the American public through the ‘Life's Simple Seven campaign’. Fundamental to the current ‘war on cancer’ is the role of lifestyle measures in primary prevention, whereby between one-third and one-half of cancers are preventable.7
Incident combined cancer rates by number of ideal health metrics: The ARIC Study, 1987–20065
| Ideal health metrics . | Sample % (n = 13 253) . | Cancer cases . | Rate/1000 years . | HR (95% CI) . |
|---|---|---|---|---|
| 0 | 2.8 | 95 | 17.3 | 1.0 (referent) |
| 1 | 15.7 | 475 | 14.3 | 0.79 (0.64–0.98) |
| 2 | 25.9 | 815 | 14.3 | 0.79 (0.64–0.98) |
| 3 | 26.3 | 779 | 13.4 | 0.74 (0.59–0.91) |
| 4 | 17.8 | 463 | 12.3 | 0.67 (0.54–0.84) |
| 5 | 8.8 | 203 | 11.3 | 0.61 (0.48–0.79) |
| 6–7 | 2.7 | 50 | 9.0 | 0.49 (0.35–0.69) |
| Ideal health metrics . | Sample % (n = 13 253) . | Cancer cases . | Rate/1000 years . | HR (95% CI) . |
|---|---|---|---|---|
| 0 | 2.8 | 95 | 17.3 | 1.0 (referent) |
| 1 | 15.7 | 475 | 14.3 | 0.79 (0.64–0.98) |
| 2 | 25.9 | 815 | 14.3 | 0.79 (0.64–0.98) |
| 3 | 26.3 | 779 | 13.4 | 0.74 (0.59–0.91) |
| 4 | 17.8 | 463 | 12.3 | 0.67 (0.54–0.84) |
| 5 | 8.8 | 203 | 11.3 | 0.61 (0.48–0.79) |
| 6–7 | 2.7 | 50 | 9.0 | 0.49 (0.35–0.69) |
Cardiovascular disease (CVD) benefit vs. cancer benefit in ARIC.5
Incident combined cancer rates by number of ideal health metrics: The ARIC Study, 1987–20065
| Ideal health metrics . | Sample % (n = 13 253) . | Cancer cases . | Rate/1000 years . | HR (95% CI) . |
|---|---|---|---|---|
| 0 | 2.8 | 95 | 17.3 | 1.0 (referent) |
| 1 | 15.7 | 475 | 14.3 | 0.79 (0.64–0.98) |
| 2 | 25.9 | 815 | 14.3 | 0.79 (0.64–0.98) |
| 3 | 26.3 | 779 | 13.4 | 0.74 (0.59–0.91) |
| 4 | 17.8 | 463 | 12.3 | 0.67 (0.54–0.84) |
| 5 | 8.8 | 203 | 11.3 | 0.61 (0.48–0.79) |
| 6–7 | 2.7 | 50 | 9.0 | 0.49 (0.35–0.69) |
| Ideal health metrics . | Sample % (n = 13 253) . | Cancer cases . | Rate/1000 years . | HR (95% CI) . |
|---|---|---|---|---|
| 0 | 2.8 | 95 | 17.3 | 1.0 (referent) |
| 1 | 15.7 | 475 | 14.3 | 0.79 (0.64–0.98) |
| 2 | 25.9 | 815 | 14.3 | 0.79 (0.64–0.98) |
| 3 | 26.3 | 779 | 13.4 | 0.74 (0.59–0.91) |
| 4 | 17.8 | 463 | 12.3 | 0.67 (0.54–0.84) |
| 5 | 8.8 | 203 | 11.3 | 0.61 (0.48–0.79) |
| 6–7 | 2.7 | 50 | 9.0 | 0.49 (0.35–0.69) |
Cardiovascular disease (CVD) benefit vs. cancer benefit in ARIC.5
The ARIC study
The ARIC (Atherosclerosis Risk In Communities) linked the ideal seven health metrics in 13 753 persons to these seven health metrics.5 The trend towards a lower cancer incidence with higher numbers of ideal health metrics was highly significant (P for trend < 0.0001).5 How was cancer incidence assessed? Incident cancer cases from 1987–2006 were obtained by linking to cancer registries. Hospital surveillance was used to identify additional cancer cases. There was an inverse, graded combined cancer incidence rate in relation to a larger number of ideal health metrics; participants with three ideal health metrics had 25% lower risk of incident cancer and participants with 6–7 ideal health metrics had >50% lower risk of incident cancer than those with no ideal health metrics (Table 1). In the proportional hazards regression model adjusting for age, sex, race, and ARIC centre, results were similar when cases of cancer occurring in the first 3 years after follow-up were removed from the analysis.
The association of ideal health metrics with new CVD was stronger than that for combined cancer. Thus, the hazard ratio for CVD comparing individuals with five ideal metrics at baseline with those with 0 was 0.18, whereas that for combined cancer was 0.61. Nonetheless, combined cancer was strongly and significantly associated with ideal health metrics, so that having ≥6 ideal health metrics was associated with a 51% reduction in cancer risk (Table 1).
Could the links between health metrics and cancer be explained solely by one of health metrics such as smoking for lung cancer? The authors conducted additional analyses removing smoking from the score of ideal health metrics and repeated the analyses for the four most common incident cancers individually. The association remained statistically significant, especially for those with ideal high health metrics of 4–6 score, representing ≈15% of the sample. Even when correcting for the relatively high levels of smoking, there was still a relationship between the numbers of health metrics in the 4–6 group, meaning that only about 25% of the ARIC population, those with a healthy lifestyle and ideal BP, blood sugar, and cholesterol, were adequately protecting themselves from both CVD and cancer.
The important overall message is that adherence to Life's Simple Seven health metrics of the American Heart Association is associated with lower incidence of total mortality, CVD, and cancer. Promotion of these ideal health metrics could reduce both CVD and cancer incidence.
Nurses' health study
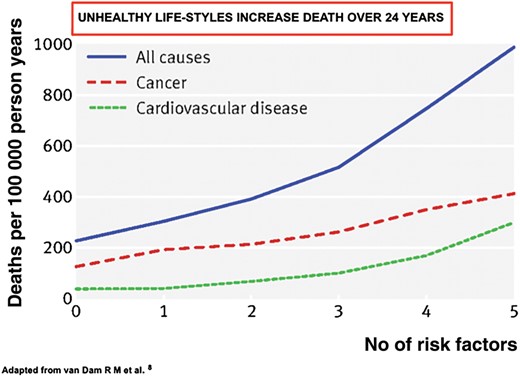
In a much larger and longer study, the Prospective Cohort study on Nurses Health over 24 years,8 77 782 US Health Professionals, analysed five adverse lifestyle factors. These were: cigarette smoking, being overweight, not taking enough moderate to vigorous physical activity, a low diet quality score, and not taking a light-to-moderate alcohol intake (the least important). A total of 8882 deaths were documented, including 1790 from cardiovascular disease and 4527 from cancer. Each lifestyle factor independently and significantly predicted mortality. Relative risks for five compared with zero lifestyle risk factors were 3.26 (95% confidence interval 2.45–4.34) for cancer mortality, 8.17 (4.96–13.47) for cardiovascular mortality, and 4.31 (3.51–5.31) for all-cause mortality. A total of 28% (25–31%) of deaths during follow-up could be attributed to smoking and 55% (47–62%) to the combination of smoking, being overweight, lack of physical activity, and a low diet quality. These results indicate that in middle-aged American women adherence to healthy lifestyle patterns was associated with markedly lower mortality from cardiovascular disease and from cancer (Figure 1).
Note the number of increased deaths from cardiovasular disease (dashed line) and deaths from cancer (middle line) with an almost five-fold increase in all-cause mortality (top line). (Figure modified from van Dam et al.8).
Links between lifestyle and cancer in Asian populations
Lifestyle and cancer in Chinese women
As lifestyle and diet are very different from those of Western nations, data from China and India become relevant. In a major study, the healthy lifestyle score based on lifestyle-related factors was independently associated with mortality outcomes (normal weight, lower waist-hip ratio, daily exercise, non-smoking, and never exposed to spouse's smoking, higher daily fruit and vegetable intake.9 Two additional factors were: body weight and no alcohol intake. A total of 71 243 women from the Shanghai Women's Health Study were followed for an average of 9 years. Compared with women with a score of zero, hazard ratios for women with seven health factors were 0.53 (0.43–0.63) for total mortality, 0.31 (0.20–0.48) for CVD mortality, and 0.76 (0.59–0.97) for cancer mortality.
A diet-cancer-cardiovascular disease relationship in India
India has very large population with differing dietary and lifestyle patterns from those found in the USA or China. A detailed case control study at Puducherry found that consumption of fats more than 30 g/day resulted in a 2.4 increased relative risk of breast cancer (CI,1.14–5.45) and consumption of oils containing more saturated fats doubled the risk (CI,1.03–4.52).10 Unfortunately, such observational studies make it difficult to establish causality between diet and cancer. As such, well-controlled population studies will be needed to establish causality, recognizing the difficulties is establishing causality between health outcomes and diet.
Possible mechanisms for links between cardiovascular disease and cancer
First, there are common predisposing factors, of which obesity is one of the best studied. Specifically, ectopic visceral fat is the link between cancer and cardiovascular disease as shown in a subpopulation of the Framingham Heart Study.11 More generally, and secondly, obesity in young adulthood is linked to diabetes in middle age,12 while diabetes is linked to cancer in that circulating markers of peripheral insulin resistance are independently associated with pancreatic cancer risk.13 Poorer prognosis in breast cancer is related to increasing levels of obesity, and metabolic dysfunction.14 Thirdly, there are links between heart failure (HF) and cancer. In a study at the Mayo Clinic, HF patients had a 68% higher risk of developing cancer [hazard ratio (1.68; 95% CI: 1.13–2.50)] adjusted for body mass index, smoking, and comorbidities.15 Fourthly, there appear to be common mechanisms at the cellular metabolic level. For example, in obesity, the circulating blood free fatty acid levels are high16 which in turn inhibits the flow of glucose through glycolysis to oxidation, while in cancer there are also inhibitions at the level of the same pathway which explains the most common metabolic hallmark of malignant tumours, i.e. the ‘Warburg effect’ (see next section).17 This effect is the propensity of cancer cells to metabolize glucose to lactic acid at a high rate even in the presence of oxygen. In the future, there may be biomarkers that could predict which patients would be likely to suffer from both CHD and cancer.18 Fifthly, the strongest links between CVD and cancer probably come from the preventative capacity of regular aspirin intake to reduce both CVD and some types of cancer and their metastases (next section).
The Warburg effect
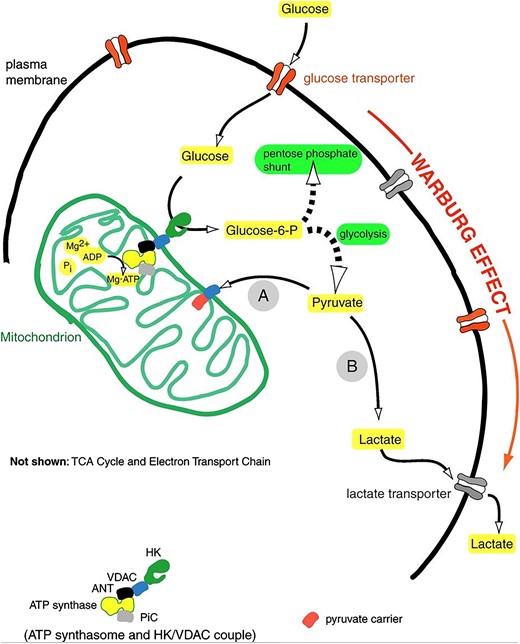
In many cardiovascular diseases, the metabolic characteristics of the cardiomyocyte mimic the metabolic characteristics of tumour cells. The normal heart has a very high energy demand which it obtains primarily from mitochondrial oxidative phosphorylation (for review see Lopaschuk et al.19). However, in many CVD disease states, mitochondrial oxidative phosphorylation is impaired and the heart switches to a more foetal metabolic profile, involving increased glycolysis as a source of energy.20 While glycolysis is increased, glucose oxidation is often impaired, especially in ischaemic heart disease and heart failure.19,21–23 This results in a scenario whereby glycolysis becomes uncoupled from glucose oxidation, resulting in the production of lactate and protons.19 This energy metabolic profile is shared with cancerous cells. The hallmark of cancer cells is a defect in mitochondrial oxidative metabolism and an increase in aerobic glycolysis compared with normal cells (for review see Boland et al.24). This uncoupling of high glycolysis rates from glucose oxidation was first observed by Otto Warburg, and is now known as the ‘Warburg Effect’ (Figure 2).25,26 While the phenotype of high glycolysis uncoupled from glucose oxidation is a phenomenon normally associated with rapidly proliferating cells (such as tumour cells), this same metabolic phenotype can also be seen in terminally differentiated cardiomyocytes from hypertrophied and failing hearts. Interestingly, this metabolic profile also occurs in vascular disorders such as pulmonary arterial hypertension (PAH) (see Sutendra and Michelakis28 for review). Mitochondrial oxidation of pyruvate derived from glycolysis is impaired in the pulmonary vasculature of PAH patients, and similar to tumour cells, glycolytic mediators such as hypoxia inducible factor-1α (HIF-1α) are also up-regulated.28
The Warburg Effect. In tumour cells, the mitochondrial metabolism of pyruvate (A) is blocked, resulting in the shunting of pyruvate to lactate (B). This is due to a decrease in mitochondrial oxidative metabolism, resulting in the aerobic production of lactate. A similar scenario can occur in many forms of cardiovascular disease, where an impaired mitochondrial oxidative metabolism of pyruvate results in an uncoupling of glycolysis from glucose oxidation. Figure from Mathupala et al.27
A key enzyme involved in the mitochondrial oxidation of glucose is pyruvate dehydrogenase (PDH). This enzyme limits the rate of glucose oxidation and is responsible for the mitochondrial decarboxylation of pyruvate to acetyl CoA.19 In turn, PDH can be phosphorylated and inhibited by PDH kinases (PDK). In ischaemic heart disease, cardiac hypertrophy and heart failure, PDKs are up-regulated and PDH is phosphorylated and inhibited.19,29,30 A similar up-regulation of PDK and inhibition of PDH also occurs in tumour cells31,32 and in the pulmonary vasculature in PAH.28,33 In addition to the up-regulation of PDK and phosphorylation of PDH, the PDH in both muscle34 and tumour cells35 also has an increase in lysine acetylation, which leads to an inhibition of PDH activity. This contributes to the inhibition of PDH and decrease in glucose oxidation seen in both diseased heart cells and tumour cells.
Since in many cardiovascular diseases mitochondrial oxidative phosphorylation is impaired, and the heart switches to a more foetal metabolic phenotype involving increased glycolysis as a source of energy,19 as also occurs in tumour cell proliferation,24 a potential therapeutic approach to treating both heart disease and cancer could involve stimulating PDH activity. In support of this, a number of studies have shown that PDK inhibition (with dichloroacetate) can improve the coupling of glycolysis to glucose oxidation and lessen ischaemic injury19,36,37 and improve heart function in heart failure.37–39 PDK inhibition (again with dichloroacetate) also decreases tumourogenesis,31,32 and improves vascular and heart function in PAH.39 As a result, stimulating mitochondrial glucose oxidation has a potential dual role in treating both cardiovascular diseases and cancer.
Aspirin and cancer: are there common mechanisms?
Aspirin and cancer prevention
Observational studies show that regular use of aspirin reduces the long-term risk of several cancers and the risk of distant metastasis. Results of methodologically rigorous studies are consistent with those obtained from randomized controlled trials.4 Benefit was apparent only after 5 years’ follow-up, and with longer duration, ≥7.5 years, solid cancers were reduced by 31% and gastrointestinal cancers by 59% (P = 0.0001). The 20-year risk of cancer death was lower in the aspirin groups. Even though this study is a retrospective analysis, in three large UK trials long-term post-trial follow-up had been obtained from death certificates and cancer registries.
Strong support for the inhibitory effect of aspirin on colorectal cancer comes from a prospective randomized study in which aspirin 600 mg daily (a dose normally not used because of the bleeding risk) for a mean of 25 months substantially reduced cancer incidence in carriers of hereditary colorectal cancer.40
In contrast, a recent meta-analysis of nine primary prevention studies claimed that there was no statistically significant effect on cancer mortality.41 The analysis involved over 100 000 participants, and covered a mean follow- up period of 6.0 years. However, effects of aspirin on cancer may take 20 years for effects on solid tumours. These authors were unable to confirm the links between aspirin and cancer reduction because cancer mortality failed to reach statistical significance even after excluding studies that had used alternate-day aspirin treatment (OR, 0.88; 95% CI, 0.76–1.01). However, it may be noted that mortality over a mean of 6 years was in the same direction as the Rothwell results. Also of note, the anti-cancer benefit of aspirin described by Algra and Rothwell4 was apparent only after 5 years’ of follow-up and became more evident the longer the follow-up. The Seshasai study had a mean follow-up time of only 6 years.41
To test the hypothesis that aspirin use is associated with a reduced risk of colorectal cancer, Bosetti et al.42 conducted a meta-analysis of all observational studies on aspirin in 12 selected cancer sites. Randomized controlled trials of aspirin,4,41 with cardiovascular events as the primary endpoint were excluded. Observational studies indicated a beneficial role of aspirin on colorectal and other digestive tract cancers; modest risk reductions were also observed for breast and prostate cancer. Regular aspirin was associated with reduced risk of colorectal cancer [confidence interval (CI) 0.67–0.79], and of other digestive tract cancers (CI = 0.50–0.76), for squamous cell esophageal cancer (CI = 0.52–0.78) for esophageal and gastric cardiac adenocarcinoma; and RR = 0.67 (CI = 0.54–0.83) for gastric cancer,
In the Lynch syndrome (a rare inherited form of non-polyposis colorectal cancer),40 aspirin 600 mg daily for 2 years of intervention (258 aspirin, 250 aspirin placebo), the hazard ratio for aspirin vs. controls was 0.41 (0.19–0.86, P = 0.02) and the incidence rate ratio was 0.37 (0.18–0.78, P = 0.008).
While aspirin has been linked to a decrease in gastrointestinal cancers, it cannot be complete discounted that some of the beneficial effects of aspirin are attributable to an earlier detection of the cancers. This may occur due to early diagnosis due to the increased incidence of bleeding with aspirin.
Mechanism of anti-cancer effect of aspirin
Colorectal cancer and atherothrombosis may share a common mechanism of disease, i.e. platelet activation in response to epithelial (in tumourigenesis) and endothelial (in tumourigenesis and atherothrombosis) injury.43 Even at low-doses, aspirin acts mainly by irreversible inactivation of platelet cyclooxygenase (COX)-1 in the presystemic circulation, which translates into a long-lasting inhibition of platelet function. Aspirin also appears to have a direct inhibitory effect on early stages of development of cancer cells. Salicylates reduced the formation of tetraploid cells from the culture of human colon carcinoma cell lines or primary mouse epithelial cells lacking tumour protein p53.44 Over half of human cancers have a loss of function of the p53 gene.45 These data explain the anti-cancer properties of p53 feedback loop.46,47
Cycloxygenase 2 expression is increased in cancers, likely due to post-translational mechanisms, and is an independent risk factor for cancer development.48,49 For instance, COX-2 overexpression is associated with worse survival among colon cancer patients.50 This effect of COX-2 on clinical outcome may be modified by p53 status.49 In addition, the cyclooxygenase product PGE2, via the EP2 PGE2 receptor, displays heightened p53 transcription and increased risk of p53 mutagenesis.50 As a result, COX-2 inhibition has the potential to decrease p53 and tumour formation.
These molecular mechanisms underlying the anti-cancer effect of aspirin suggest that aspirin, in addition to being taken for cardiovascular protection, may have potential anti-cancer effects. However, further studies are needed to determine if the benefits of aspirin in cancer prevention outweigh the potential side effects of aspirin (such as increased risk of bleeding). There are no data to recommend a specific starting age. Arguably, in those persons middle-aged and beyond, the combined anti-cancer and vascular protective benefits of aspirin outweigh the relatively small risks of a serious gastrointestinal bleed with aspirin.
Links between cardiovascular disease and cancer in patients using statins
Statins reduce heart attacks and also certain types of cancer such as carcinoma of the prostate.17,27,51 The links probably extend much further, in that Nielsen and Nordestgaard studied the relationship between statin use (prior to cancer diagnosis) and cancer-related mortality in the entire Danish population from 1995 to 2009 in adults >40 years of age.17 In 18 721 statin users and 277 204 statin non-users, they found improved survival with statin exposure for 13 cancers, including the four most common cancers: lung (HR: 0.87; CI: 0.83–0.92), colorectal (HR: 0.79; CI: 0.75–0.85), prostate (HR: 0.81; CI: 0.75–0.88), and breast (HR: 0.88; CI: 0.80–0.99). The strict design of the study adjusted for multiple confounding factors, yet unfortunately no allowance was made for the carcinogenic effects of smoking which was not recorded in the Danish data base, although expected to be very low. The basic protective mechanism could include statin-mediated down-regulation of the mevalonate pathway. Studies have suggested that suppression of mevalonate synthesis depletes tumour tissues of two intermediate products, farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are critical in cell growth.52
Statins reduce progression of prostate cancer, the second most common malignancy in men worldwide51 but by which mechanism is unknown. If bone marrow stroma, PC-3 cells were isolated from patients and the binding, invasion, and colony formation of these was assessed by in vitro co-cultures in the presence of different statins. The statins that acted directly on PC-3 cells were the lipophilic statins (atorvastatin, mevastatin, and simvastatin), but not the non-lipophilic pravastatin. There were reductions both in number (P = 0.0055) and size (P = 0.0019) of colonies formed within the isolated bone marrow stroma.51
Summary
There are increasing links between cardiovascular disease and cancer, starting with optimal lifestyle, including diet, and extending to statin-induced reductions in the incidences of both cardiovascular disease and cancer. Furthermore, there are plausible mechanisms that are being explored.
Funding
L.H.O. was supported by the University of Cape Town and G.D.L. by the University of Alberta.
Conflict of interest: none declared.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.