-
PDF
- Split View
-
Views
-
Cite
Cite
Claude Daubert, Nathalie Behar, Raphaël P. Martins, Philippe Mabo, Christophe Leclercq, Avoiding non-responders to cardiac resynchronization therapy: a practical guide, European Heart Journal, Volume 38, Issue 19, 14 May 2017, Pages 1463–1472, https://doi.org/10.1093/eurheartj/ehw270
Close - Share Icon Share
Abstract
Over two decades after the introduction of cardiac resynchronization therapy (CRT) into clinical practice, ∼30% of candidates continue to fail to respond to this highly effective treatment of drug-refractory heart failure (HF). Since the causes of this non-response (NR) are multifactorial, it will require multidisciplinary efforts to overcome. Progress has, thus far, been slowed by several factors, ranging from a lack of consensus regarding the definition of NR and technological limitations to the delivery of therapy. We critically review the various endpoints that have been used in landmark clinical trials of CRT, and the variability in response rates that has been observed as a result of these different investigational designs, different sample populations enrolled and different means of therapy delivered, including new means of multisite and left ventricular endocardial simulation. Precise recommendations are offered regarding the optimal device programming, use of telemonitoring and optimization of management of HF. Potentially reversible causes of NR to CRT are reviewed, with emphasis on loss of biventricular stimulation due to competing arrhythmias. The prevention of NR to CRT is essential to improve the overall performance of this treatment and lower its risk–benefit ratio. These objectives require collaborative efforts by the HF team, the electrophysiologists and the cardiac imaging experts.
Introduction
Cardiac resynchronization therapy (CRT) is a highly effective but still underused treatment of the desynchronized failing heart.1,2 Despite being cost-effective,3–5 CRT is relatively expensive, especially when combined with an implantable cardioverter defibrillator (ICD). Like all invasive therapies, it exposes to risks of procedural failures reaching 13% in recently published trials,6–9 and of complications in 17% of patients at 6 months in the Danish registry.10 In addition, ∼30% of system recipients do not benefit from CRT, though the proportions of non-responders vary depending on the definition and criteria applied.1,11,12 Non-response to therapy has remained the fixed Achilles' heel of CRT over the years, offering considerable medical and financial challenges, such that efforts must be pursued to clarify and resolve its determinants. It is certainly multifactorial and may involve multiple pre-, peri-, and post-implant factors.13 Identifying its causes and, eventually, improving its results require a structured, multidisciplinary approach.13,14
This review discusses (i) the definition of CRT response and a quest for a consensus and (ii) the means available to prevent non-response (NR) to therapy, including (a) judicious patient selection, (b) optimal pacing configuration, device programming, and management of heart failure (HF), (c) remote monitoring (RM), and (d) the management of associated conditions that may interfere with the delivery of therapy.
The key strategies to prevent, identify, and manage non-responders are listed in Table 1.
Prevention of non-response to cardiac resynchronization therapy
| Non-response . | ||||
|---|---|---|---|---|
| Prevention . | Detection . | Managementa . | ||
| Pre-implant | Thoughtful patient selection Guidelines indications | Primary diagnosis Consensus definition Response/NR Multidisciplinary approach Attending staff Heart failure team Heart failure status Electrophysiologists Device interrogations Cardiac imaging | Advanced heart failure |
|
| Implant | Optimal stimulation configuration Right ventricular lead LV lead: maximum delay Multipolar LV stimulation | Suboptimal device programming | Device re-programming Atrioventricular/interventricular intervals Stimulation Mode Rate Output | |
| Device settings Nominal Automatic Individual | Lead(s) Failure Improper position | Reoperationa for lead(s) Revision(s) Repositioning Addition → MSP | ||
| Post-implant | Remote monitoring—optimization of care Uptitration of pharmaceuticals Non-pharmacological interventions: Education Exercise training Heart failure monitoring | Concomitant disorders Arrhythmias Atrial fibrillation Atrial tachycardia Ventricular | Antiarrhythmic drugs Catheter ablationa Atrial fibrillation Atrioventricular node Ventricular extrasystoles | |
| Mitral regurgitation | Treatment of MRa | |||
| Myocardial ischaemia | Revascularizationa | |||
| Non-response . | ||||
|---|---|---|---|---|
| Prevention . | Detection . | Managementa . | ||
| Pre-implant | Thoughtful patient selection Guidelines indications | Primary diagnosis Consensus definition Response/NR Multidisciplinary approach Attending staff Heart failure team Heart failure status Electrophysiologists Device interrogations Cardiac imaging | Advanced heart failure |
|
| Implant | Optimal stimulation configuration Right ventricular lead LV lead: maximum delay Multipolar LV stimulation | Suboptimal device programming | Device re-programming Atrioventricular/interventricular intervals Stimulation Mode Rate Output | |
| Device settings Nominal Automatic Individual | Lead(s) Failure Improper position | Reoperationa for lead(s) Revision(s) Repositioning Addition → MSP | ||
| Post-implant | Remote monitoring—optimization of care Uptitration of pharmaceuticals Non-pharmacological interventions: Education Exercise training Heart failure monitoring | Concomitant disorders Arrhythmias Atrial fibrillation Atrial tachycardia Ventricular | Antiarrhythmic drugs Catheter ablationa Atrial fibrillation Atrioventricular node Ventricular extrasystoles | |
| Mitral regurgitation | Treatment of MRa | |||
| Myocardial ischaemia | Revascularizationa | |||
aAll interventional choices require a careful risk/benefit evaluation.
Prevention of non-response to cardiac resynchronization therapy
| Non-response . | ||||
|---|---|---|---|---|
| Prevention . | Detection . | Managementa . | ||
| Pre-implant | Thoughtful patient selection Guidelines indications | Primary diagnosis Consensus definition Response/NR Multidisciplinary approach Attending staff Heart failure team Heart failure status Electrophysiologists Device interrogations Cardiac imaging | Advanced heart failure |
|
| Implant | Optimal stimulation configuration Right ventricular lead LV lead: maximum delay Multipolar LV stimulation | Suboptimal device programming | Device re-programming Atrioventricular/interventricular intervals Stimulation Mode Rate Output | |
| Device settings Nominal Automatic Individual | Lead(s) Failure Improper position | Reoperationa for lead(s) Revision(s) Repositioning Addition → MSP | ||
| Post-implant | Remote monitoring—optimization of care Uptitration of pharmaceuticals Non-pharmacological interventions: Education Exercise training Heart failure monitoring | Concomitant disorders Arrhythmias Atrial fibrillation Atrial tachycardia Ventricular | Antiarrhythmic drugs Catheter ablationa Atrial fibrillation Atrioventricular node Ventricular extrasystoles | |
| Mitral regurgitation | Treatment of MRa | |||
| Myocardial ischaemia | Revascularizationa | |||
| Non-response . | ||||
|---|---|---|---|---|
| Prevention . | Detection . | Managementa . | ||
| Pre-implant | Thoughtful patient selection Guidelines indications | Primary diagnosis Consensus definition Response/NR Multidisciplinary approach Attending staff Heart failure team Heart failure status Electrophysiologists Device interrogations Cardiac imaging | Advanced heart failure |
|
| Implant | Optimal stimulation configuration Right ventricular lead LV lead: maximum delay Multipolar LV stimulation | Suboptimal device programming | Device re-programming Atrioventricular/interventricular intervals Stimulation Mode Rate Output | |
| Device settings Nominal Automatic Individual | Lead(s) Failure Improper position | Reoperationa for lead(s) Revision(s) Repositioning Addition → MSP | ||
| Post-implant | Remote monitoring—optimization of care Uptitration of pharmaceuticals Non-pharmacological interventions: Education Exercise training Heart failure monitoring | Concomitant disorders Arrhythmias Atrial fibrillation Atrial tachycardia Ventricular | Antiarrhythmic drugs Catheter ablationa Atrial fibrillation Atrioventricular node Ventricular extrasystoles | |
| Mitral regurgitation | Treatment of MRa | |||
| Myocardial ischaemia | Revascularizationa | |||
aAll interventional choices require a careful risk/benefit evaluation.
Definition of response/non-response: a need for consensus
The main goals of HF treatment are relief of symptoms, restoration of quality of life (QOL), slowing of disease progression, lower the rates of emergency room visits and hospitalizations, and prolong life. Alleviation of symptoms and lower morbidity are the main objectives for patients in New York Heart Association (NYHA) HF functional Classes III and IV, and preventing disease and HF progression is priority for patients in NYHA Classes I and II.
Despite 20 years of clinical development, a consensus definition of response and NR to CRT has not been reached. The definitions adopted in randomized trials vs. in clinical practice remain dissimilar. Clinical trials measure typically event-based endpoints, while less well-defined criteria are used to assess responses in practice. Definition of response to CRT is also a matter of expectation. Patients presenting with severe HF seek symptom relief and better QOL, first. However, once this has been achieved with CRT the expectations evolve toward less frequent hospitalizations, greater social exposure, and longer survival.1
Measures of response to cardiac resynchronization therapy
Functional and quality-of-life measures
An assessment of multiple components of well-being, including symptoms, QOL, and functional capacity, in order to demonstrate a consistency of effect, is preferred over a single arbitrary indicator, such NYHA functional class.15 Cardiopulmonary exercise testing is a useful pathophysiologic exploration, though requires high expertise and is time-consuming, thus impractical for large clinical trials or for the clinic setting.
Event-based measures
Measures of CRT response that include all-cause mortality, inevitably include events unrelated to CRT. However, it is the most unbiased means of measuring the effects of CRT on mortality. The outcome of HF hospitalization (number of unplanned hospitalizations or total number of days spent in the hospital for management of HF decompensation) is suitable to monitor the effect of CRT on the HF status, though it can be biased, especially in open-label clinical trials.
Remodelling measures
Echocardiographic measures of reverse remodelling have been abundantly used to assess the CRT response, alone in mechanistic studies or as a secondary endpoint.17 A significant decrease in left ventricular (LV) dimensions, with or without increase in ejection fraction (EF), defines a positive response with threshold values of 15–25% for LV end-systolic volume index.17,18 There is general consensus to examine reverse remodelling after ≥6 months of CRT, when the process has stabilized.18,19 Comparative studies showed no correlation between echocardiographic and clinical response.17
Composite measures
Composite endpoints are often used in clinical trials of CRT. They are only reliable when each component is of similar importance, or one component of the endpoint (usually death) precludes the attainment of other components. Composite endpoints should not be used to inflate event rates (by adding blood tests or imaging in the composite for statistical purpose8) and should be clinically meaningful. Among them, Packer's clinical composite response20 has the advantage to have been validated in landmark CRT trials.16,21 It explores almost all components of HF therapy, includes patient self-assessment and classifies patients as worsened, unchanged, or improved.
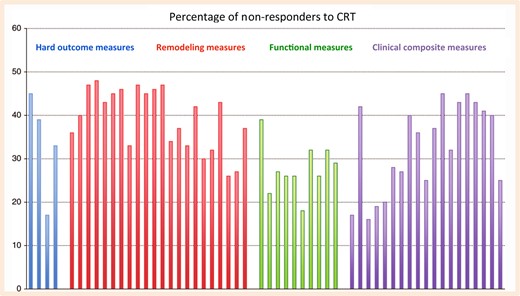
Variable response rates depending on the measures used
Rates of non-response to cardiac resynchronization therapy depending on the measure used in controlled trials and large observational studies of cardiac resynchronization therapy, each represented by a bar. Event-based measures are shown as blue, remodelling measures as red, functional and quality of life measures as green, and composite endpoints as purple bars. Adapted from Daubert et al.1 Reproduced with permission from Europace and the authors.
A plea for a consensus in the definition of response vs. non-response to cardiac resynchronization therapy
These observations beg for a clinically more meaningful consensus in the definition of response vs. NR to CRT. Composite measures are needed to encompass all aspects of the therapeutic response. We could propose to define a positive response as being alive with a sustained improvement in well-being, including measures of HF morbidity for patients presenting with moderate or severe HF, and being alive with no sign of disease progression for patients presenting with less advanced HF. Freedom from major post-operative complications should be an additional component in all patients. The composite score proposed by Packer,20 or an equivalent scheme, is suitable to evaluate the clinical response to therapy. In patients presenting with mild HF, remodelling should be included in the global composite score.
Patient selection
In professional practice guidelines, CRT is indicated to lower the morbidity and mortality of patients presenting with symptomatic HF, a depressed left ventricular ejection fraction (LVEF) and evidence of ventricular dyssynchrony by a prolonged QRS complex.24,25 The absence of any reversible cause of HF like ischaemia, arrhythmia (tachycardia-induced cardiomyopathy), or primary valvular disease must be checked before discussing implantation. Guidelines also indicate that the QRS morphology is an important predictor of the therapeutic response2–23 and underscore the recommendation for patients presenting with typical left bundle branch block, though these recommendations are based on subgroup analyses of CRT trials.24–26 It is noteworthy that the landmark CRT trials did not use QRS morphology as an inclusion criterion. A single, large, and randomized trial used it to define subgroups prospectively.26 The European and North American guidelines, nevertheless, formulated nearly similar and concordant recommendations and grading (Table 2). Cardiac resynchronization therapy is not indicated for patients presenting with a QRS duration <120 ms.2,23
Indications for cardiac resynchronization therapy in patients presenting with heart failure, sinus rhythm, and spontaneous conduction
| Class of recommendation . | Level of evidence . | Baseline electrocardiographic characteristics . |
|---|---|---|
| I | A | Left bundle branch block with >150 ms QRS duration |
| I | B | Left bundle branch block with QRS duration between 120 and 150 ms |
| IIa | B | No left bundle branch block and QRS duration > 150 ms |
| IIb | B | No left bundle branch block and QRS duration between 120 and 150 ms |
| Class of recommendation . | Level of evidence . | Baseline electrocardiographic characteristics . |
|---|---|---|
| I | A | Left bundle branch block with >150 ms QRS duration |
| I | B | Left bundle branch block with QRS duration between 120 and 150 ms |
| IIa | B | No left bundle branch block and QRS duration > 150 ms |
| IIb | B | No left bundle branch block and QRS duration between 120 and 150 ms |
Guidelines of European Society of Cardiology and American College of Cardiology/American Heart Association 2013.
Indications for cardiac resynchronization therapy in patients presenting with heart failure, sinus rhythm, and spontaneous conduction
| Class of recommendation . | Level of evidence . | Baseline electrocardiographic characteristics . |
|---|---|---|
| I | A | Left bundle branch block with >150 ms QRS duration |
| I | B | Left bundle branch block with QRS duration between 120 and 150 ms |
| IIa | B | No left bundle branch block and QRS duration > 150 ms |
| IIb | B | No left bundle branch block and QRS duration between 120 and 150 ms |
| Class of recommendation . | Level of evidence . | Baseline electrocardiographic characteristics . |
|---|---|---|
| I | A | Left bundle branch block with >150 ms QRS duration |
| I | B | Left bundle branch block with QRS duration between 120 and 150 ms |
| IIa | B | No left bundle branch block and QRS duration > 150 ms |
| IIb | B | No left bundle branch block and QRS duration between 120 and 150 ms |
Guidelines of European Society of Cardiology and American College of Cardiology/American Heart Association 2013.
Beyond the simple surface ECG, other techniques have been used to assess ventricular dyssynchrony with the aim to improve prediction of response. The value of LV mechanical dyssynchrony based on current imaging techniques remains uncertain so that it is not recommended as a selection criterion for CRT.1,2 In the future, new technologies such as non-invasive electrocardiographic mapping,27 multimodality imaging,1 or combination of both are expected to improve dyssynchrony evaluation and perhaps patient selection.
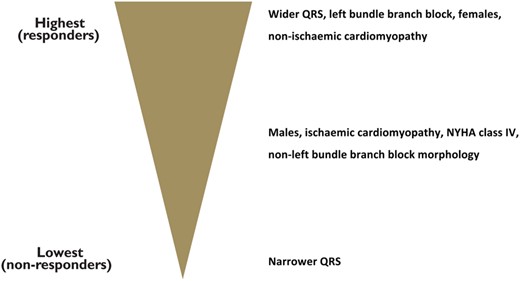
Factors influencing the response to cardiac resynchronization therapy in patients presenting in sinus rhythm and spontaneous conduction. Adapted from Brignole et al.2 Reproduced with permission from the European Heart Journal and the authors.
In unselected patient populations, 25% of CRT recipients are in permanent atrial fibrillation (AF).30 These patients are older and suffer from more advanced HF and higher prevalence of comorbidities than patients who are in sinus rhythm. These patients are probably not eligible for AF ablation. Because they have not been systematically studied prospectively, candidacy for CRT is less clearly defined. A single, large, randomized trial stratified its enrolled sample population by presence vs. absence of AF.31 In the predefined subgroup of 229 patients with AF, representing 12.7% of the randomized sample, CRT conferred no apparent benefit, in contrast to the 25% lower risk of death or hospitalization for management of HF observed in the overall population. It is particularly noteworthy, however, that 50% of the patients in AF had per cent biventricular (BiV) stimulation >90%. Based on data from registries, and by analogy with patients in sinus rhythm, the practice guidelines issued by the European Society of Cardiology (ESC) recommend to consider CRT for patients in permanent AF, whose intrinsic QRS duration is ≥120 ms and LVEF <35%, and who remain in NYHA HF functional Class III or ambulatory Class IV despite optimal medical treatment, provided that ‘BiV pacing as close to 100% as possible can be achieved’ (Class IIa indication, level of evidence B). This recommendation must be strengthen by further evidence. Ablation of the atrioventricular (AV) junction may be needed to achieve this objective.2 Another recommendation is for AF patients who are candidates to AV junction ablation for rate control because of severe symptoms; for guidelines, CRT should be considered in patients with reduced LVEF.2
A stronger consensus has been reached for patients already implanted with a standard device who suffer from worsening HF in presence of a LVEF <35% and a high percentage of right ventricular (RV) pacing irrespective of QRS width. These patients should be offered an upgrade to CRT (Class I indication, level of evidence B).2 However, individual decisions must take into consideration the up to 23% risk of complications observed in prospective registries.10–32De novo CRT implants in patients with standard pacemaker indications has been evaluated in two large randomized trials. The BLOCK-HF includes patients with Class I or IIa indications for pacing, Classes I–III HF, and an LVEF <50%.8 After a 3-year follow-up, the composite endpoint of death from all causes and HF events was reduced by 26% in the group assigned to BiV pacing compared with the group assigned to standard RV pacing. BIOPACE was designed as a mortality-morbidity trial in patients with chronic symptomatic AV block and a mean LVEF of 55%.9 After a 5.7-year follow-up, no significant difference was observed between BiV stimulation and standard pacing. At this time, CRT indication should be limited to patients presenting with HF, a depressed LVEF and a high expected percentage of ventricular pacing.2
In recent surveys of the unselected use of CRT, the rate of off-label device implants (which are associated with poor outcomes) remains >20%.33 In such patients, turning-off CRT could be an option in case of worsening symptoms.1 One may reasonably expect a lower rate of NR to therapy by a higher compliance to the professional practice guidelines.
Optimal therapy delivery
Pacing configuration
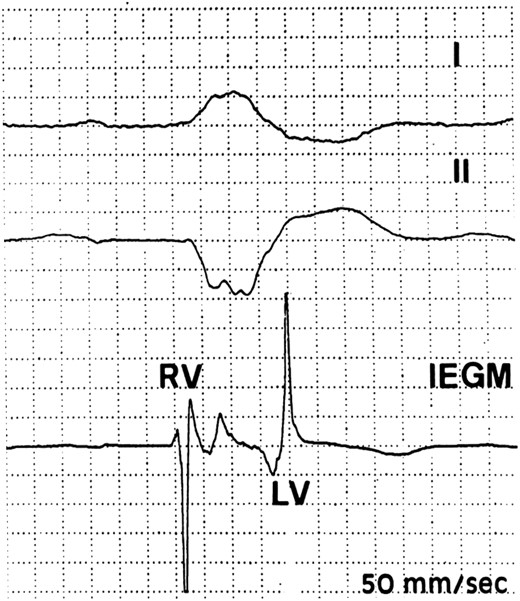
Perioperative identification of earliest and latest activation sites during spontaneous conduction. In this patient with typical left bundle branch block, the earliest site was in the right ventricular mid-septum and the latest at the left ventricular base in the proximal segment of a posterolateral vein. The 190 ms interventricular conduction delay was longer than the 175 ms QRS duration on the surface electrocardiogram.
Right ventricular lead
The RV lead has usually been implanted at the RV apex. Non-apical implants are expected to activate both ventricles more physiologically. A meta-analysis of randomized studies comparing apical and non-apical leads positions in pacemaker recipients suggests that septal or RV outflow tract pacing preserves LV function more effectively, particularly in presence of a depressed LVEF.36 The safety and efficacy of septal implants has been confirmed with ICD leads.37 In recipients of CRT systems, post hoc analyses of large trials revealed no significant difference between septal and apical RV locations with respect to the degree of cardiac remodelling and incidence of clinical endpoints.38,39 The SEPTAL-CRT trial was designed to compare the LV remodelling associated with RV apical vs. septal lead locations.40 That randomized, multicentre trial confirmed the non-inferiority of a septal compared with an RV apical location. Whether a location of the RV lead chosen according to the implant of the LV lead, with an optimization of the leads separation as well as the delay in impulse propagation, improves the outcomes of CRT needs further studies.
Left ventricular lead
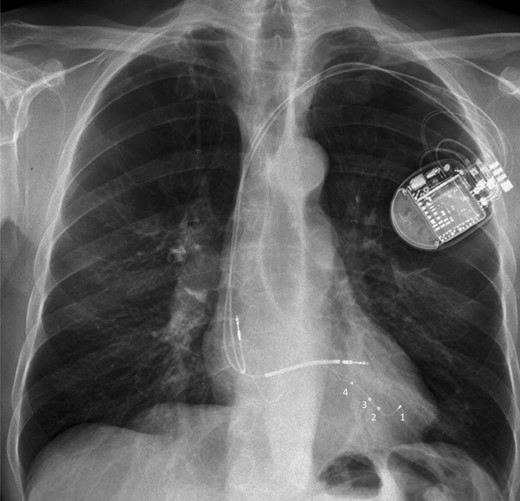
Chest X-ray in antero-posterior view showing a quadripolar LV lead with the distal tip placed at the apex to provide more lead stability. To prevent apical pacing, left ventricular pacing can be delivered at dipoles 2–3 or 3–4 closer to the left ventricular base, or by using other pacing vectors with the right ventricular coil depending on pacing thresholds and the acute haemodynamic response when assessed.
The merit of targeting the site of latest mechanical or electrical activation has been extensively examined. In two randomized studies using pre-operative speckle-tracking echocardiography, similar net clinical benefits were obtained by implanting the LV lead near the site of latest mechanical activation.43,44 However, this imaging technique is time-consuming and its reproducibility uncertain. A more practical and useful intra-operative measurement is the delay between QRS onset on the surface ECG (or the intracardiac RV electrogram) and the LV electrogram. Stimulation at the site of the longest delay confers clinical benefits, including alleviation of symptoms, and lower rates of hospitalizations for management of HF and of cardiac remodelling.45,46 These observations support the targeting of the site of maximum LV delay as guide to positioning of the LV lead.2
Left ventricular endocardial pacing
Stimulation of the LV endocardium seems promising because of the faster propagation of the electrical wavefront at the endocardium than the epicardium.47 Derval et al. found that LV endocardial stimulation improved haemodynamic function significantly, while the optimal stimulation site was variable among patients.48 A single multicentre study examined the feasibility and safety of LV endocardial pacing via the atrial transseptal approach.49 While the success rate was 90%, several strokes and transient ischaemic attacks were observed. This technique requires long-term anticoagulation to prevent thromboembolisms and may disturb mitral valve function. Furthermore, the management of infections may require cardiac surgery to extract the LV lead. This means of stimulation remains under clinical investigation and should not be used routinely.
Multisite pacing
Other stimulation schemes have been proposed with a view to obtain a more rapid and homogeneous LV activation. Multisite pacing (MSP) has been tested in several configurations. Small pilot studies observed a trend toward greater reverse remodelling with an additional LV or RV lead.50,51 Multisite pacing, using two LV leads, was tested in a randomized study of patients who had not responded to standard BiV stimulation. No clinical or echocardiographic benefits were found and a high complication rate, infections in particular was observed.52 In practice, MSP remains under clinical investigation and should not be used routinely.
Multipoint pacing is new way to deliver MSP through a unique quadripolar LV lead and a dedicated algorithm enabling (i) LV stimulation from two separate dipoles located in the same CS tributary and (ii) RV stimulation. In short-term haemodynamic studies, LV dP/dt was greater than that with standard BiV stimulation.53 Based on the results of a small randomized study, which observed a significant decrease in the rate of non-responders with multipoint stimulation,54 a large randomized trial based on LV reverse remodelling in non-responders to CRT <ClinicalTrials.gov NCT 02006069> was recently launched, with a follow-up of 6 months.
Practical recommendations
The ESC practice guidelines recommend to implant the LV lead via the CS, avoiding an apical location.2 The selection of a posterolateral or lateral target vein remains often based on anatomical factors, while an optimum position can be reached by targeting the region of latest mechanical or electrical activation or both. Targeting the longest LV activation time intra-operatively is readily feasible and likely to increase the rate of response to CRT. The guidelines offer no precise recommendation for the RV lead implant.
Suboptimal stimulation due to a suboptimal LV lead position, insufficient ventricular resynchronization, loss of LV capture, and other factors is a common cause of NR to CRT.13 Because of the high rate of complications associated with reoperations,10,32 the programming should be optimized at first attempt (see below). In recipients of quadripolar leads and pulse generators capable of delivering multipoint stimulation, this option could be considered despite limited clinical evidence in its support. If necessary and with a risk–benefit ratio apparently favourable, a reoperation can be considered, with a view to re-implant the LV lead to an optimal site or to add an another ventricular lead.
Device programming
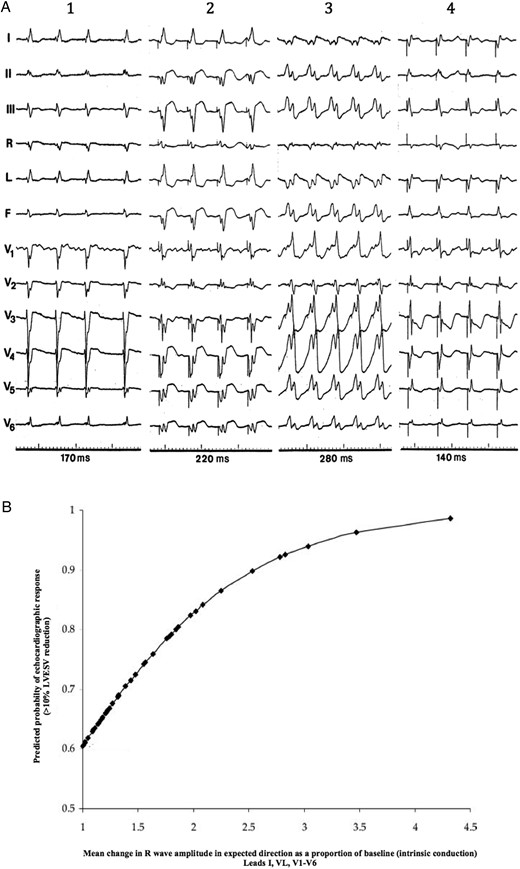
(A) Typical electrocardiogram changes after cardiac resynchronization therapy in a hyper-responder patient. Compared with intrinsic conduction (1), right ventricular pacing (2) and left ventricular pacing (3) biventricular pacing (4) causes a significant shortening of QRS simultaneously emerge a dominant-R wave in lead V1, both signs indicating optimal fusion between left ventricular pre-excitation and intrinsic conduction. (B) Correlation between changes in R-wave amplitude in lead V1 after cardiac resynchronization therapy, and the probability of echocardiographic response defined as >10% reduction in left ventricular endsystolic volume at 6 months. Greater changes in R-wave amplitude after cardiac resynchronization therapy, indicative of wavefront fusion, predict higher probability of response. From Sweeney et al.60 Reproduced with permission of authors and editor.
The most important settings to achieve an optimal cardiac resynchronization are the pacing mode, the lower and upper rate limits, the capture output, the stimulation vectors configuration, and the AV–VV intervals. The programming of a slow backup rate is recommended, to limit unnecessary atrial pacing.1 The upper tracking rate must be programmed sufficiently high to prevent a loss of BiV stimulation during exercise.1 Rate responsive stimulation in patients with severe atrial chronotropic incompetence must be programmed cautiously in order to not stress a failing heart. A safety margin for the LV output must be programmed to guarantee a consistent BiV capture, though new-generation devices are equipped with an autocapture function.
Observational studies have identified suboptimal programming of the AV–VV delays as determinant factor of a poor response to CRT.13 Doppler echocardiography is the reference method of AV and VV intervals optimization.61 Until recently, this time-consuming technique was widely used. However, the results of large multicentre trials suggested that this routine echocardiographic optimization is mostly ineffective, compared with empiric programming of a 100–120 ms sensed AV delay and simultaneous BiV stimulation.62,63 In the same trials, the non-inferiority of algorithms of automatic adjustment based on electrical timings, compared with empiric programming, was not confirmed. Promising new algorithms creating an optimal fusion between spontaneous conduction and LV stimulation,64 or using a haemodynamic sensor,65 need further investigations.
In summary, the systematic routine optimization of the AV and VV delays in all CRT system recipients is not warranted. The ESC guidelines recommend to first programme a fixed 100–120 ms AV delay without VV interval.2 However, in subgroups of patients, especially in the presence of a long interatrial delay, the intervals should be optimized after the implant. Further echocardiographic evaluations and optimizations are advised in case of NR to CRT.
Remote monitoring
Device monitoring
Several large randomized trials of various types of devices, including pacemakers, ICD, and CRT-D, conducted in various countries, have consistently shown that the replacement of routine ambulatory visits by RM lowers the rate of follow-up appointments and safely enables the early detection of actionable events.66 However, until recently, no study had shown a significant decrease in the rates of death or hospitalizations for management of cardiovascular events conferred by RM. In-TIME is the first randomized trial, which examined the effects of RM in recipients of CRT-D or dual-chamber ICD.21 At 1 year, RM was associated with a significant decrease in the rate of worsened clinical composite score and death from all causes, suggesting that by improving the clinical outcomes in this high-risk population, it can participate in the prevention of NR to CRT. Remote monitoring enables a close monitoring of the per cent of BiV stimulation, AF episodes, and frequent ventricular extrasystoles (VES), which may interfere with the success of CRT. These observations support the offering of RM to all recipients of CRT systems as part of their routine follow-up.2
Telemonitoring of heart failure
Telemonitoring by implanted electronic devices enables the surveillance of multiple variables, including body weight, blood pressure, heart rate and its variability, thoracic impedance, pulmonary artery pressure, left atrial pressure, and amount of daily activity. Several trials have examined the merits of managing remotely patients suffering from HF by telemonitoring of one or several of these variables.67 An analysis of pooled data suggested that a score based on multiple variables recorded by the device could identify patients at increased risk of hospitalization for management of HF within 30 days.68 However, when all data were pooled the clinical value of HF telemonitoring could not be verified because of inconsistent results. Therefore, no recommendation can be currently formulated with regard to the primary prevention of the NR to CRT by HF RM.69
Optimization of heart failure management
Optimization of care is a goal pursued for all patients suffering from HF, when they do not respond to CRT in particular.1 It includes the optimization of drug therapy, education, exercise training programmes,70 and monitoring.69 Optimal care is best provided by a multidisciplinary programme, in which the CRT recipients are urged to participate. The doses of pharmaceuticals recommended in the professional practice guidelines are often limited by hypotension and renal dysfunction. Cardiac resynchronization therapy, however, supports the systemic blood pressure and heart rate, enabling an increase in the doses of β-adrenergic blockers without risk of profound bradycardia or hypotension.71
Eliminating potential reversible causes of non-response
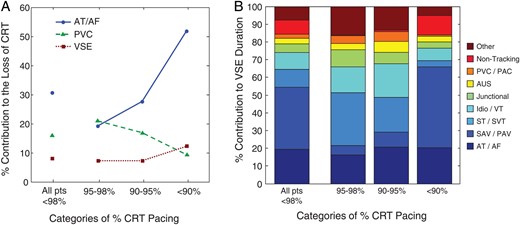
(A) Contributions to the loss of stimulation plotted according to the percentage of cardiac resynchronization therapy stimulation loss (>98%, 95–98%, 90–95%, and <90%). The contributors were atrial tachyarrhythmias, ventricular extrasystoles, and ventricular sensing episodes. From Cheng et al.72; with permission from the authors and from Circulation: Arrhythmia Electrophysiology. (B) Contributors plotted according to the severity of cardiac resynchronization therapy stimulation loss, including atrial tachyarrhythmias, ventricular extrasystoles, and loss of ≥10 consecutive cycles of cardiac resynchronization therapy stimulation as captured in ventricular sensing episodes. Right panel: Reasons for ventricular sensing episodes plotted according to the severity of cardiac resynchronization therapy pacing loss: premature ventricular contraction/premature atrial contraction; AUS, atrial undersensing with appropriate ventricular sensing; JR, junctional rhythm; Idio/VT, idioventricular rhythm, ventricular tachyarrhythmia, or ventricular oversensing; ST/SVT, sinus tachycardia or supraventricular tachycardia; sensed atrioventricular/paced atrioventricular interval, intrinsic atrioventricular conduction faster than the programmed sensed or paced atrioventricular delays; atrial tachycardia/atrial fibrillation, ≥10 consistent beats without cardiac resynchronization therapy that occurred during atrial tachycardia/atrial fibrillation.
Atrial tachyarrhythmias
Atrial fibrillation and atrial tachycardias contribute to a suboptimal response to CRT by (i) their rapid rates inhibiting the stimulation, (ii) the irregularity of the rhythm, and (iii) loss of atrial function. In recent studies that interrogated the device memories, the cumulative incidence of new onset ATA ranged between 7% in patients with mild HF,73 and 30% in patients suffering from more advanced HF.74–77 The incidence was twice higher in patients with histories of ATA.74 The prognostic value of AF in CRT systems recipients remains uncertain. In MADIT CRT, the clinical benefit conferred by CRT-D compared with ICD alone was not diminished by a history of intermittent ATA or by their development during the trial.73 In contrast, in a large Italian registry, >10 min episodes of ATA detected by the devices were associated with an increased risk of NR to therapy and of death or hospitalization for the management of HF.77 These discordant observations are attributed to different study designs, patient populations, and paroxysmal vs. non-paroxysmal ATA.
The suppression of AF in presence of HF and a depressed LVEF remains a challenge, for which guidelines continue to recommend amiodarone as first-line therapy.78 When unsuccessful or contraindicated, catheter ablation may be considered.78,79 A review of controlled trials and observational studies including 1838 patients presenting with LV systolic dysfunction who underwent AF ablation found that (i) sinus rhythm was maintained in 60% of patients at a mean follow-up of 23 months, (ii) the overall complication rate was 4.2%, and (iii) LVEF increased by a mean of 13%.80 It is noteworthy that the absence of structural heart disease, suggesting that the presence of a tachycardia-induced cardiomyopathy, and a shorter interval between the diagnosis of HF and the ablation procedure predicted better outcomes. In contrast, CRT patients, who by definition present with structural heart disease, a profoundly depressed LVEF and a long history of HF are likely to not benefit from AF ablation.
The results of four randomized clinical trials published thus far are less favourable.81–84 After 6–12 months of follow-up, LVEF increased by 5–10% in patients free from AF recurrence after catheter ablation. The success rates without antiarrhythmic therapy, rates of repeat procedure, and complication rates were 50–84, 19–54, and 8–15%, respectively. These observations suggest that the likelihood of staying in sinus rhythm after AF ablation is lower in patients presenting with HF, and that the rates of procedure-related complications are higher. In summary, AF ablation may improve LV function in selected patients suffering from HF and treated in highly experienced centres78 but specific data are lacking in CRT HF patients.
Ventricular extrasystoles
Frequent VES or NSVTs may worsen or cause LV dysfunction85 and, in selected patients, ablation of extrasystolic foci may restore LV function.86,87 European Society of Cardiology guidelines indicate that amiodarone or catheter ablation should be considered in patients with frequent symptomatic VESs or NSVTs, or if VESs or NSVTs contribute to reduced LVEF (Class IIa, level of evidence B).88 In CRT patients, frequent VES with or without fusion or pseudofusion may markedly interfere with BiV stimulation and contribute to a NR to therapy.13 The value of VES catheter ablation was assessed in a study of 65 non-responders to CRT (defined as the absence of clinical improvement and <10% decrease in LV end-systolic volume after 1 year of therapy) with >10 000 VES/24 h. The short- and long-term success rates were 91 and 88%, respectively.89 Major complications occurred in two patients. At 1 year of follow-up, mean LVEF increased by 7% and LV end-systolic volume and NYHA functional class decreased by 18% and by 1 point, respectively. Coupled with guidelines recommendations, these preliminary results suggest that VES ablation is an option for selected non-responder patients treated in highly experienced medical centres.
Secondary valve regurgitation
Mitral regurgitation
Secondary MR due to desynchronized LV contraction and cavity dilatation is common in CRT recipients. In CRT trials, the prevalence of ≥grade 2 MR at baseline ranged between 15% in patients in NYHA functional Class I or II6,16 and 40% in patients in NYHA functional Class III or IV.28,90 After implant or CRT systems, MR decreases, stabilizes, and rarely increases. In randomized trials, the severity index of MR decreased consistently to nearly 40% with CRT, remaining unchanged without CRT.90 In large observational studies, a decrease in MR by ≥1 grade was observed in up to over 60% of patients with moderate-to-severe MR at baseline.91,92 When persistent, the severity of MR measured at 3 months was an independent predictor of unfavourable outcome.28 The professional practice guidelines have issued two Class IIb recommendations (level of evidence C) for the management of secondary, chronic MR: (i) mitral valve surgery may be considered in patients with severe MR, an LVEF >30%, and who remain symptomatic despite optimal management, including CRT, and (ii) the percutaneous mitral clip procedure might be an alternative for eligible patients based on echocardiographic criteria, and who are inoperable or at high surgical risk.93 Since most CRT system recipients are at high surgical risk, the mitral clips were proposed for patients who remained in NYHA functional Class III or IV and with persistent moderate or severe MR for ≥6 months, despite CRT. In a study of over 50 severely symptomatic non-responders to CRT, the perioperative mortality was 8% and at 6 months of follow-up, 85% were alive, MR decreased to grade 1 or 2 in 80%, 65% were in NYHA functional Class I or II, LV end-diastolic volume had decreased by a mean of 10% and LVEF increased by a mean of 7%.94 These observations suggest that mitral clips may contribute to the management of non-responders to CRT who present with severe, persistent secondary MR.
Tricuspid regurgitation
Most candidates for CRT present with baseline tricuspid regurgitation, the severity of which is an independent predictor of mortality.95 Its worsening during CRT in 15% of patients independently predicts poor clinical and echocardiographic responses. The unknown merits of correcting severe functional tricuspid regurgitation to improve the response to CRT are an issue that will be addressed with the development of new percutaneous tricuspid valve therapies.96
Myocardial ischaemia
Among CRT system recipients, >50% suffer from ischaemic cardiomyopathy. Based on expert consensus, guidelines recommend (Class IIa, LOE C): (i) non-invasive imaging to detect myocardial ischaemia and viability in patients with known CAD presenting with de novo HF24; this recommendation can be reasonably extended as pre-implant work up to CAD patients eligible for CRT, (ii) coronary angiography when ischaemia may be contributing to HF or myocardial viability exists97 in patients eligible for revascularization, (iii) revascularization by CABG or alternatively PCI if suitable coronary anatomy.98 These recommendations are mainly based on the STICH trial, which missed the primary endpoint of all-cause mortality, though revealed a trend toward a decrease in the rates of cardiovascular and all-cause deaths and HF hospitalization.99 They apply to the management of CRT system recipients presenting with coronary artery disease. In absence of conclusive studies, the role played by ischaemia in the NR phenomenon and the preventive role of coronary revascularization are unknown.
Conclusion
The prevention of NR to CRT is essential to improve its overall performance and lower its risk–benefit ratio. This objective requires collaborative efforts by the HF team, the electrophysiologists, and the cardiac imaging experts. A programme to prevent NRs to CRT includes judicious patient selection, optimal therapy delivery, and the use of RM. The identification of non-responders must be based on a consensus definition and be timely. Once the cause(s) identified, the care of non-responders must be individualized.
Authors’ contributions
J.-C.D., N.B., C.L. drafted the manuscript. R.M., P.M. made critical revision of the manuscript for key intellectual content.
Conflict of interest: C.D. received Consultancy fee from LivaNova, Medtronic Inc. and ST Jude Medical. N.B. received Consultancy fee from Boston Scientific. R.P.M. received Consultancy fee from LivaNova. P.M. received Consultancy fees from LivaNova, Medtronic Inc., Boston Scientific; Research grants from LivaNova, Biotronik; Speaker fees from Livanova, St Jude Medical. C.L. received Consultancy fee from Medtronic, St Jude Medical. Speaker’s fee from Medtronic, St Jude Medical; Livanova, Medtronic Inc., Boston Scientific.




Comments