-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Goette, Ruediger C. Braun-Dullaeus, Atrial fibrillation is associated with impaired cognitive function and hippocampal atrophy: silent cerebral ischaemia vs. Alzheimer's disease?, European Heart Journal, Volume 29, Issue 17, September 2008, Pages 2067–2069, https://doi.org/10.1093/eurheartj/ehn343
Close - Share Icon Share
Atrial fibrillation (AF) is the most common cause of thromboembolic stroke. Several studies have shown an activation of the plasmatic and cellular clotting system in patients with AF.1,2 Interestingly, these changes occur within hours after initiation of AF. In order to predict the individual risk for AF-related thromboembolic events, the CHADS2-score has been developed.1 It takes into account that especially elderly patients, patients with diabetes, hypertension, heart failure, and/or previous stroke are at greatest risk. Depending on the CHADS2-score, the yearly risk of stroke may vary between 1.9% (CHADS2-score of 0) and 18.2% (CHADS2-score of 6). The pathophysiological basis of how these clinical risk factors affect cardiac thrombogenesis or predict future thromboembolic events is complex. According to the Virchow triad there are three determinants of thrombus formation: circulatory stasis, hypercoagulable state, and endothelial injury at the site of thrombus initiation. Clinical co-morbidities such as diabetes, hypertension, or heart failure influence all three factors of Virchow's triad. Increased expression of adhesion molecules at the atrial endocardium promotes the adhesion of leukocytes, leading to the formation of granulocyte–platelet conjugates at the endocardium.3 This process appears to be intensified in heart failure. Diabetes and hypertension further increase inflammatory responses. Thus, small granulocyte–platelet conjugates or larger thrombi may develop in fibrillating atria, causing silent cerebral ischaemia or manifest stroke.2,4,5 In addition to thromboembolic events, cerebral hypoperfusion induced by cardiac beat-to-beat variability is considered as an alternative pathophysiological mechanism for silent cerebral ischaemia during AF.6 Thus, it appears very reasonable to believe that AF may cause multiple episodes of cerebral ischaemia (clinically manifest or silent) leading to impaired cognitive function and vascular dementia in the long term. Many cross-sectional studies support this assumption.7–9 The first large longitudinal study in patients with and without AF showed that dementia is more common following the diagnosis of AF. Miyasaka et al. followed 2837 subjects diagnosed with first AF for almost 5 years.10 They showed that the rate of dementia was 2.7% at 1 year and 10.5% at 5 years in this cohort. However, several studies demonstrated that the association between AF and cognitive impairment is independent of stroke and other cardiovascular risk factors. Thus, the simplistic sequence of repetitive microemboli, cognitive decline, and vascular dementia does not fully explain the association between AF and dementia. Interestingly, AF was identified as a risk factor for Alzheimer's disease.11 Alzheimer's disease causes accumulation of abnormally folded β-amyloid and tau proteins. Amyloid fibrils form cerebral plaques, which exert cytotoxic effects leading to gross atrophy of the affected brain regions. Similar to the amyloidogenesis in Alzheimer's disease, the development of amyloid fibrils and amyloid deposits, which are formed from misfolded atrial natriuretic peptides, are present in the atria of elderly patients with AF.12
Knecht et al. report on the cognitive decline in patients with AF.13 Their present study included a total of 685 patients: 122 with AF and 563 serving as controls. Patients with a history of stroke, dementia, and depression were excluded from the study. Extensive neuropsychological testing was performed to assess learning and memory, working memory, executive function, and attention, as well as visuospatial abilities. Patients also underwent 3-Tesla magnetic resonance imaging (MRI) to assess territorial brain infarctions and white matter lesions. After adjustment for age, gender, education, and cardiovascular risk factors, the authors could show that AF is associated with worse learning and memory function, whereas other cognitive functions were not affected. Corresponding to the impairment in memory function, MRI revealed hippocampal atrophy, whereas there was no correlation between AF and total brain volume. Interestingly, there was no clear difference between patients with persistent and paroxysmal AF. Of note, white matter lesions (sign of microangiopathy) were not different in patients with and without AF. Thus, the present study suggests that AF is associated with specific alterations in brain morphology and function. This finding may argue against the hypothesis that AF causes cognitive decline and potentially vascular dementia by multiple subclinical thromboembolic brain infarctions, since such lesions would affect all perfused areas of the brain diffusely. In addition, vascular dementia affects executive functioning and visuospatial skills more often than learning and memory. Thus, can the present results be interpreted in a way that AF induces an anatomically distinct cerebral lesion? This is a fascinating hypothesis (Figure 1). Nevertheless, as stated by the authors, the hippocampus appears to be one of the most sensitive structures of the brain. Alterations are described to occur in Alzheimer's disease, hypoxia, brain trauma, and metabolic abnormalities. Thus, the association between AF and hippocampal atrophy might be due to the fact that the present study selected patients at a relatively young age. Therefore, the reported hippocampal changes might just reflect early brain alterations in the time course of AF regardless of the underlying mechanism. The present study also has several other limitations. The study included a limited number of patients, particularly with AF. Cognitive function is influenced by multiple variables, which are hard to balance in small patient cohorts. Therefore, the present findings have to be validated in larger cohorts. This is of particular importance since the association between AF and dementia was not shown consistently in previous studies.14,15 In addition, the present cross-sectional study does not allow any conclusions to be drawn with regard to the impact of AF therapy on cerebral changes. It is intriguing to speculate that AF therapy including rhythm control and anticoagulation protects against hippocampal atrophy. Nevertheless, the pathophysiological interactions of various metabolic and cardiovascular diseases with cognitive function are highly complex, and therefore it might not be easy to demonstrate clear causal relations. In the present study, the use of anticoagulation therapy was negatively associated with performance in learning and memory. Does this mean that this kind of therapy increases the risk of cognitive dysfunction or is the present result just due to a sampling bias including a subgroup of elderly patients with multiple co-morbidities, who were characterized by treatment with anticoagulants? Thus, longitudinal studies encompassing repetitive brain imaging and detailed cardiac rhythm monitoring are needed to answer the most important questions in this regard, such as the time course of cerebral changes, the impact of different types of AF on cerebral lesions, or potential therapeutic effects. Such studies also have to focus on principle questions: is the occurrence of AF followed by cerebral alterations or is AF secondarily due to autonomic dysbalance induced by localized brain atrophy? It is also tempting to speculate that AF and Alzheimer's disease share a common link with regard to protein misfolding and amyloidgenesis in different organs. All these issues are not answered, yet.
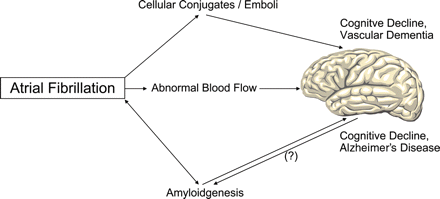
Association between atrial fibrillation (AF), cognitive decline, and dementia. AF increases the occurrence of circulating cellular conjugates (e.g. leukocyte–platelet conjugates) and the development of atrial thrombi. Cerebral embolization of the factors impairs cognitive function. In addition, AF induces alterations in cerebral blood flow, which may lead to cerebral hypoperfusion. AF has been described as a risk factor for the occurrence of Alzheimer's disease. Amyloid fibrils occur in Alzheimer's disease as well as in subgroups of patients with AF, although the fibril proteins are different. So far it is unknown if both diseases share a common pathophysiological basis with regard to amyloidgenesis.
Nevertheless, the present study is important because it provides first data about the occurrence of localized cerebral changes associated with loss of function in specific cognitive domains during AF. Thus, the present study may stimulate even more detailed studies on cognitive testing and morphological brain analyses in patients with and without AF. It may open a window for close and intense interactions between neurologists, radiologists, and cardiologists dealing with the clinical problem of ‘atrial fibrillation’.
Conflict of interest: none declared.
References
References
The above article uses a new reference style being piloted by the EHJ that shall soon be used for all articles.
doi:10.1093/eurheartj/ehn341
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.